Hydrogen + Oxygen `to` Water
Calcium oxide + Carbon dioxide `to` Calcium carbonate Both the reactions are combination reactions as two substances are combining to form a new substance.
Silver nitrate + Copper `to` Copper nitrate + Silver
Copper is displacing silver from its salt solution, hence it is al single displacement reaction.
Sodium nitrate `to` Sodium nitrite + Oxygen
Sodium nitrate is decomposing into sodium nitrite and oxygen hence, it is a decomposition reaction.
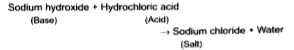
Acid and base combine to form salt and water hence, it is a neutralisation reaction which is exothermic in nature.