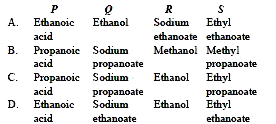A compound 'P' having molecular formula, `C_3 H_6 O_2` reacts with Na metal to form a compound 'Q' and evolves a gas which burns with a pop sound. Compound 'P' on treatment with an alcohol 'R' in presence of an acid forms a sweet smelling compound 'S' having molecular formula, `C_5 H_(10) O_2`. On addition of NaOH to 'P', it gives 'Q' and water. 'S' on treatment with NaOH solution gives back 'Q' and 'R'.
Identify P, Q, R and S.