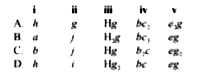The given part of the modern periodic table shows positions of elements a to j.

Fill in the blanks by choosing an appropriate option.
Element ___i___ resembles sodium in properties and element ___ii___. belongs to the same group as nitrogen. The formula of the hydride of g is ____iii_____. The formula of compound formed between b and c is ___iv___ while the formula of compound formed between e and g is ___v____.