P, Q, R, S, T and U are six consecutive elements of the periodic table. The given table shows the formulae of the oxides of these elements.
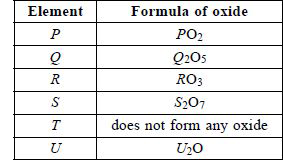
Read the given statements carefully.
I. Element belongs to group 15 and period 3.
II. The elements P and S form a covalent compound of type `PS_(4)`
III. Element R belongs to group 14 and period 2.
IV. The elements U and S form an ionic compound of type US
The correct statement(s) is/are.