Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A monoatomic gas is taken from A to B through two different processes ...
Text Solution
|
- A certain mass of is taken from an initial thermodynamics state A to a...
Text Solution
|
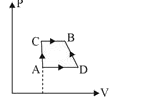
- A gas can be ken from A to B via two different processes ACB and ADB. ...
Text Solution
|
- An ideal gas follows a cyclic process as shown in figure. Internal ene...
Text Solution
|
- In thermodynamic process pressure of a fixedmass of gas is changed in ...
Text Solution
|
- A gas can be taken from A to B via two different processes ACB and ADB...
Text Solution
|
- A gas can be taken from A to B via two different processes ACB and DB....
Text Solution
|
- एक गैस को अवस्था A से B में दो भिन्न प्रक्रमों ACB तथा ADB द्वा...
Text Solution
|
- A gas can be ken from A to B via two different processes ACB and ADB. ...
Text Solution
|