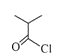A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- (A) overset(NH3)to(B) underset(KOH//Br2)overset(KOBr)to CH3 - overset(...
Text Solution
|
- Among the following structures I to IV :- C2 H5-overset(CH3)overset("|...
Text Solution
|
- IUPAC nomenclature of CH3-underset(CH2-CH3)underset(|)overset(CH3)over...
Text Solution
|
- In the given reaction CH3 - overset(OH) overset(| ) (CH)- underset(C...
Text Solution
|
- Which of the following tertiary (3^@) Is oriented (a) CH3-underset(CH3...
Text Solution
|
- With reference to the following structures (A) CH3-underset(CH3)unders...
Text Solution
|
- CH3-C -= C-CH3 underset("Catalyst")overset(H2-"Lindlar's")to A unders...
Text Solution
|
- CH2 = CH - CH = underset(CH3)underset(|)C-CH3 overset(+HBr)to CH3-over...
Text Solution
|
- Give the IUPAC names of following amines. CH3-CH2-overset(CH3)overset...
Text Solution
|