Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
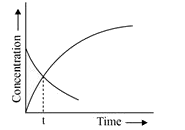
- Consider the following first order decomposition process An to nA ...
Text Solution
|
- Consider the following first order decomposition and the accompanying ...
Text Solution
|
- The rate constant for a first order reaction is 60s^(-1). How much tim...
Text Solution
|
- The rate constant for a first order reaction is 60s^(-1). How much tim...
Text Solution
|
- Plot a graph of concentration of a reactant at time t, [ (a-x)] agains...
Text Solution
|
- Consider the following first order decomposition process: Here, ''t'' ...
Text Solution
|
- Which of the following is/are correct for the first order reaction ? (...
Text Solution
|
- The decomposition of a substance follows first order kinetics. If its ...
Text Solution
|
- The rate constant for a first order reaction is 60s^(-1) . How much ti...
Text Solution
|