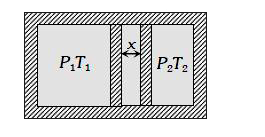
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (ISOBARIC AND ISOCHORIC PROCESS)|10 VideosTHERMODYNAMICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (HEAT ENGINE, REFRIGERATOR AND SECOND LAW OF THERMODYNAMICS)|35 VideosTHERMODYNAMICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (ISOTHERMAL PROCESS)|16 VideosSURFACE TENSION
ERRORLESS|Exercise ASSERTION & REASON|11 VideosTHERMOMETRY, THERMAL EXPANSION AND CALORIMETRY
ERRORLESS|Exercise ASSERTION & REASON|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THERMODYNAMICS-NCERT BASED QUESTIONS (ADIABATIC PROCESS)
- An ideal gas follows a process described by PV^(2)=C from (P(1), V(1),...
Text Solution
|
- One mole of a monoatomic ideal gas is expanded by a process described ...
Text Solution
|
- Following figure shows on adiabatic cylindrical container of volume V(...
Text Solution
|
- Pressure-temperature relationship for an ideal gas undergoing adiabati...
Text Solution
|
- 1mm^3 of a gas is compressed at 1 atmospheric pressure and temperature...
Text Solution
|
- An ideal gas at pressure of 1 atmosphere and temperature of 27^(@)C is...
Text Solution
|
- The ratio of adiabatic bulk modulus and isothermal bulk modulus of a g...
Text Solution
|
- A diatomic gas initially at 18^(@) is compressed adiabatically to one-...
Text Solution
|
- An ideal gas at 27^(@)C is compressed adiabatically to 8//27 of its or...
Text Solution
|
- The temperature of a hypothetical gas increases to sqrt(2) times when ...
Text Solution
|
- The state of an ideal gas was changed isobarically. The graph depicts ...
Text Solution
|
- The bulk modulus of a gas is defined as B = -VdP/dV. For an adiabatic ...
Text Solution
|
- The work done in an adiabatic change in a gas depends only on
Text Solution
|
- One mole of an ideal gas at an initial temperature true of TK does 6R ...
Text Solution
|
- One mole of an ideal gas with gamma=1.4, is adiabatically compressed s...
Text Solution
|
- Ideal gas undergoes an adiabatic change in its state from (P1 V1 , T1...
Text Solution
|
- A gas expands with temperature according to the relation V = kT^(2//3)...
Text Solution
|
- The internal energy of the gas increases In
Text Solution
|
- In changing the state of a gas adiabatically from an equilibrium state...
Text Solution
|
- During an adiabatic expansion, the increase in volume is associated wi...
Text Solution
|