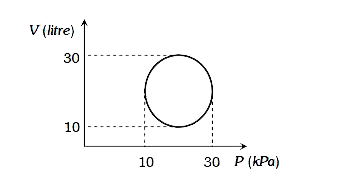
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
ERRORLESS|Exercise ASSERTION AND REASON|20 VideosTHERMODYNAMICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (HEAT ENGINE, REFRIGERATOR AND SECOND LAW OF THERMODYNAMICS)|35 VideosSURFACE TENSION
ERRORLESS|Exercise ASSERTION & REASON|11 VideosTHERMOMETRY, THERMAL EXPANSION AND CALORIMETRY
ERRORLESS|Exercise ASSERTION & REASON|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THERMODYNAMICS-PAST YEARS QUESTIONS
- A thermodynamic process is shown in Fig. The pressures and volumes cor...
Text Solution
|
- In (figure). shows two path that may be taken by a gas to go from a st...
Text Solution
|
- Heat energy absorbed by a system in going through a cyclic process sho...
Text Solution
|
- A sample of ideal monoatomic gas is taken round the cycle ABCA as show...
Text Solution
|
- A system is taken from state a to state c by two paths adc and abc as ...
Text Solution
|
- A sample of 0.1 g of water of 100^(@)C and normal pressure (1.013 xx 1...
Text Solution
|
- An ideal gas A and a real gas B have their volumes increases from V to...
Text Solution
|
- Air in a cylinder is suddenly compressed by a piston, which is then ma...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- In the given (V - T) diagram, what is the relation between pressure P1...
Text Solution
|
- A gas is compressed isothermally to half its initial volume. The same ...
Text Solution
|
- 4.0 g of a gas occupies 22.4 litres at NTP. The specific heat capacity...
Text Solution
|
- In which of the following processes, heat is neither absorbed nor rele...
Text Solution
|
- A sample of gas expands from volume V(1) to V(2). The amount of work d...
Text Solution
|
- Thermodynamic processes are indicated in the following diagram
Text Solution
|
- A Carnot engine, having an efficiency of eta=1//10 as heat engine, is ...
Text Solution
|
- Two Carnot engines are operated in succession. The first engine receiv...
Text Solution
|
- The cofficient of performance of a refrigerator is 5. If the temperatu...
Text Solution
|
- A refrigerator works between 4^(@)C and 30^(@)C. It is required to rem...
Text Solution
|
- The temperature inside a refrigerator is t2 °C and the room temperatur...
Text Solution
|