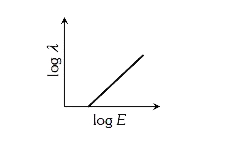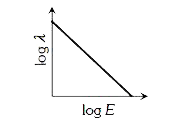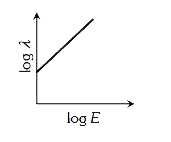A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTRON, PHOTON, PHOTOELECTRIC EFFECT & X -RAY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Photon and Photoelectric Effect)|78 VideosELECTRON, PHOTON, PHOTOELECTRIC EFFECT & X -RAY
ERRORLESS|Exercise NCERT BASED QUESTIONS (X-Rays)|34 VideosELECTRON, PHOTON, PHOTOELECTRIC EFFECT & X -RAY
ERRORLESS|Exercise ASSERTION & REASON|27 VideosELECTROMAGNETIC INDUCTION
ERRORLESS|Exercise ASSERTION & REASON|20 VideosELECTRONICS
ERRORLESS|Exercise Assertion & Reason|26 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ELECTRON, PHOTON, PHOTOELECTRIC EFFECT & X -RAY-NCERT BASED QUESTIONS (Matter Waves)
- lambda(e),lambda(p) and lambda(alpha) are the de-Broglie wavelength of...
Text Solution
|
- A proton and and alpha - particle are accelerated through a potential ...
Text Solution
|
- The wavelength of matter wave is independent of
Text Solution
|
- The linear momentum of an electron, initially at rest, accelerated thr...
Text Solution
|
- The ratio of the de-Broglie wavelengths of an electron of energy 10 eV...
Text Solution
|
- If a proton and electron have the same de Broglie wavelength, then
Text Solution
|
- Two large parallel plates are connected with the terminal of 100 V pow...
Text Solution
|
- A proton, a neutron, an electron and an alpha-particle have same energ...
Text Solution
|
- An electron is moving with an initial velocity vecv=v0hati and is in a...
Text Solution
|
- Find the ratio of kinetic energy of the particle to the energy of the ...
Text Solution
|
- According to de broglie , the de broglie wavelength for electron in an...
Text Solution
|
- An alpha-particle moves in curcular path of radius 0.83 cm in the pres...
Text Solution
|
- Consider the four gases hydrogen, oxygen, nitrogen and helium at the s...
Text Solution
|
- If m is the mass of an electron and c is the speed of light, the ratio...
Text Solution
|
- A particle is dropped from a height H. The de-Broglie wavelength of th...
Text Solution
|
- An electron (mass m) with an initial velocity v=v(0)hati is in an elec...
Text Solution
|
- Photons of energy 7 eV are incident on two metals A and B with work fu...
Text Solution
|
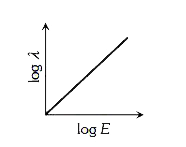
- The graph between the energy log E of an electron and its de-Broglie ...
Text Solution
|
- Consider figure. Suppose the voltage applied to A is increased. The di...
Text Solution
|
- The de-Broglie wavelengh of a neutron in thermal equilibrium with heav...
Text Solution
|