A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTRON, PHOTON, PHOTOELECTRIC EFFECT & X -RAY
ERRORLESS|Exercise PAST YEARS QUESTIONS|71 VideosELECTRON, PHOTON, PHOTOELECTRIC EFFECT & X -RAY
ERRORLESS|Exercise ASSERTION & REASON|27 VideosELECTRON, PHOTON, PHOTOELECTRIC EFFECT & X -RAY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Photon and Photoelectric Effect)|78 VideosELECTROMAGNETIC INDUCTION
ERRORLESS|Exercise ASSERTION & REASON|20 VideosELECTRONICS
ERRORLESS|Exercise Assertion & Reason|26 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ELECTRON, PHOTON, PHOTOELECTRIC EFFECT & X -RAY-NCERT BASED QUESTIONS (X-Rays)
- The ratio of the energy of an X - ray photon of wavelength 1 Å to that...
Text Solution
|
- The minimum wavelength of X-ray emitted by X-rays tube is 0.4125 Å. Th...
Text Solution
|
- Which one of the following statements is WRONG in the context of X-ray...
Text Solution
|
- Electrons with de-Broglie wavelength lamda fall on the target in an X-...
Text Solution
|
- The fig. represents the observed intensity of X-rays emitted by an X-r...
Text Solution
|
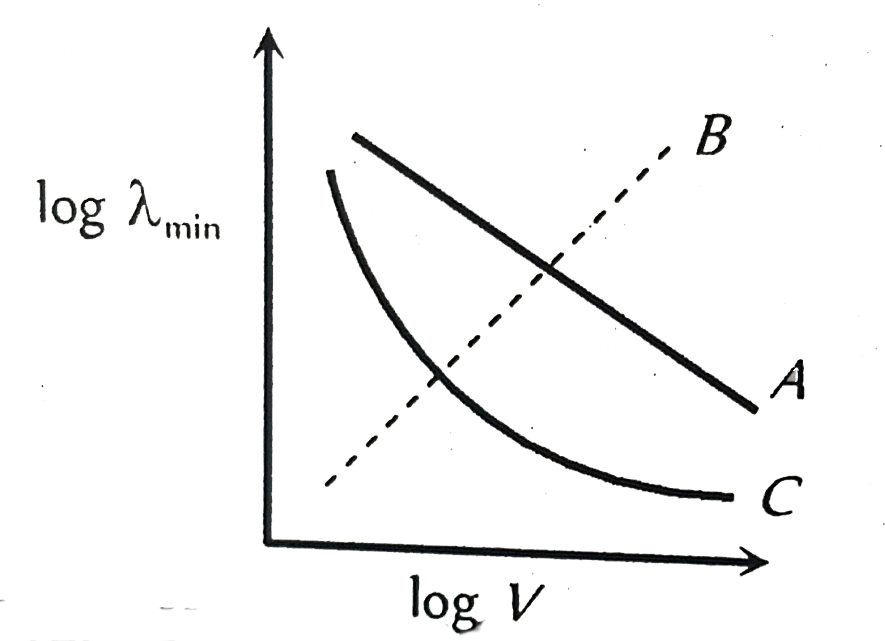
- The dependence of the short wavelength limit lamda(min) on the accele...
Text Solution
|
- Moseley's law relates the frequencies of line X-arays with the followi...
Text Solution
|
- Two elements A and B with atomic numbers Z(A) and Z(B) are used to pro...
Text Solution
|
- X-rays are produced by accelerating electrons by voltage V and let the...
Text Solution
|
- The variation of wavelength lamda of the Kalpha line with atomic num...
Text Solution
|
- The graph that correctly represents the relation of frequency v of a ...
Text Solution
|
- The intensity distribution of X-rays from two Coolidge tubes operated ...
Text Solution
|
- The graph between the square root of the frequency of a specific line ...
Text Solution
|
- For the production of characteristic K(gamma), x-ray, the electron tra...
Text Solution
|
- The X-ray wavelength of L(alpha) line of platinum (Z=78) is 1.30Å. The...
Text Solution
|
- Let lambda(alpha), lambda(beta),and lambda'(alpha) denote the wavelen...
Text Solution
|
- In X-ray tube when the accelerating voltage V is halved.the difference...
Text Solution
|
- A photon of wavelength lamda is absorbed by an electron confined to a...
Text Solution
|
- In the diagram a graph between the intensity of X-rays emitted by a mo...
Text Solution
|
- The longest wavelength that can be analysed by a sodium chloride cryst...
Text Solution
|