A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC AND NUCLEAR PHYSICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Nucleus, Nuclear Reaction)|57 VideosATOMIC AND NUCLEAR PHYSICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Radioactivity)|78 VideosALTERNATING CURRENT
ERRORLESS|Exercise Assertion & Reason|13 VideosCURRENT ELECTRICITY
ERRORLESS|Exercise ASSERTION AND REASON|26 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ATOMIC AND NUCLEAR PHYSICS-Assertion and Reason
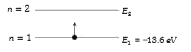
- In the nth orbit, the energy of an electron E(n)= -( 13.6)/(n^(2)) eV ...
Text Solution
|
- It is not possible to use .^35 C 1 as the fuel for fusion energy. Th...
Text Solution
|
- .^90 Sr from the radioactive fall out from nuclear bomb ends up in the...
Text Solution
|
- Assertion : Neutrons penetrate matter more readily as compared to prot...
Text Solution
|
- Assertion: Bohr had to postulate that the electrons in stationary orbi...
Text Solution
|
- Radioactive nuclei emit beta^-1 particles. Electrons exist inside th...
Text Solution
|
- Assertion: ""(Z)X^(A) undergoes 2alpha- decays, 2beta- decays and 2gam...
Text Solution
|
- Density of all the nuclei is same. Radius of nucleus is directly pro...
Text Solution
|
- Assertion : Isobars are the element having same mass number but diffe...
Text Solution
|
- Assertion: The force of repulsion between atomic nucleus and alpha-par...
Text Solution
|
- Assertion: The positively changed nucleus of an atom has a radius of a...
Text Solution
|
- Assertion: According to classical theory, the proposed path of an elec...
Text Solution
|
- Assertion: Electrons in the atom are held due to coulomb forces. Rea...
Text Solution
|
- The mass of a nucleus can be either less than or more than the sum of ...
Text Solution
|
- Assertion: Hydrogen atom consists of anly one electron but its emissio...
Text Solution
|
- Assertion Bamer series lies in the visble region of electromagnetic s...
Text Solution
|
- Assertion: For the scattering of alpha-particles at a large angles, on...
Text Solution
|
- Cabalt-60 is useful in cancer therapy. Cabalt-60 is source of Y-radi...
Text Solution
|
- Amongst alpha,beta and gamma-particles, alpha-particle has maximum pen...
Text Solution
|
- The ionising power of alpha-particle is less compared to alpha-particl...
Text Solution
|
- The mass of beta-particles when they are emitted is higher than the ma...
Text Solution
|