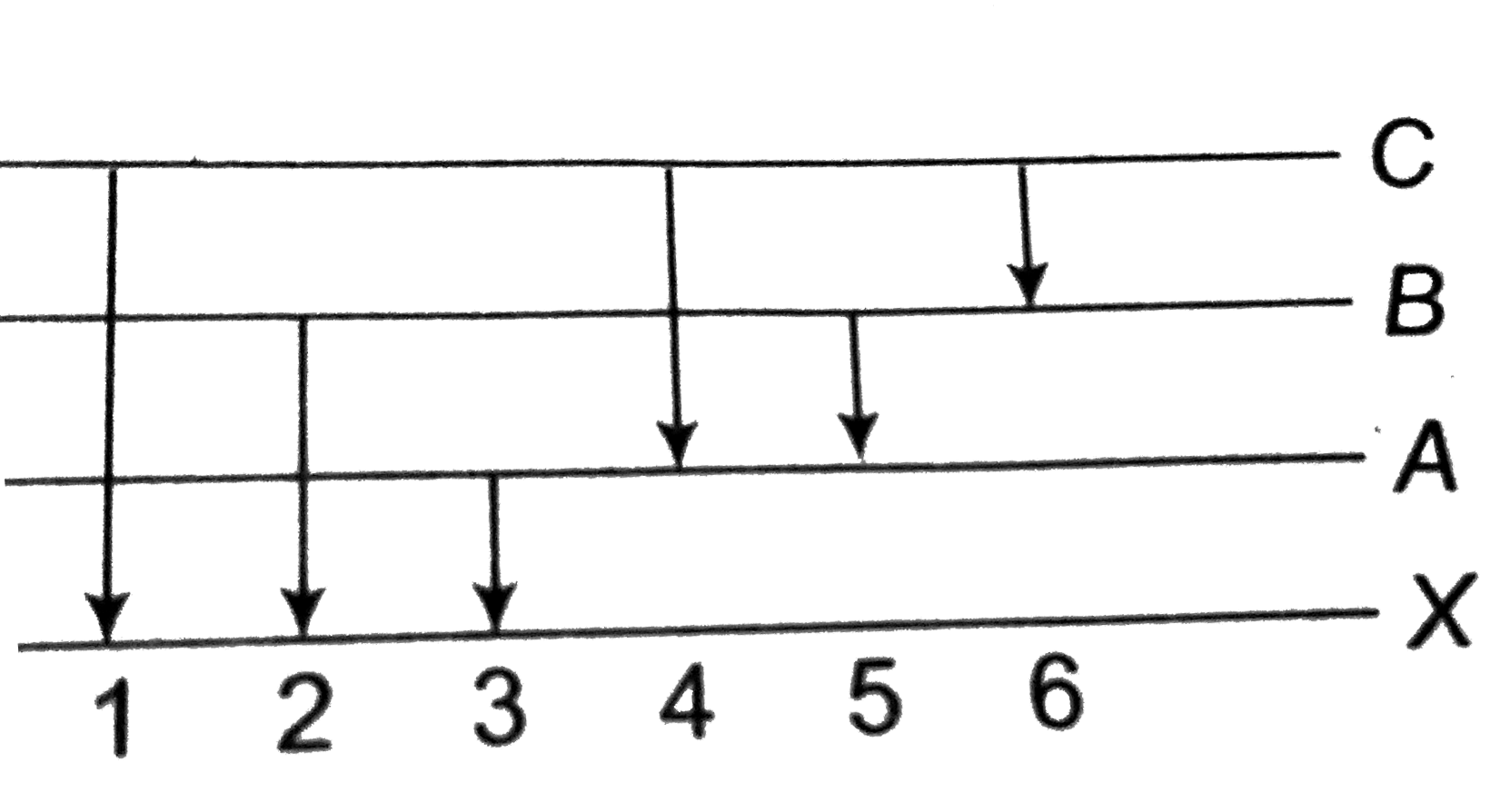
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC AND NUCLEAR PHYSICS
ERRORLESS|Exercise Assertion and Reason|23 VideosATOMIC AND NUCLEAR PHYSICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Radioactivity)|78 VideosALTERNATING CURRENT
ERRORLESS|Exercise Assertion & Reason|13 VideosCURRENT ELECTRICITY
ERRORLESS|Exercise ASSERTION AND REASON|26 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ATOMIC AND NUCLEAR PHYSICS-Past Years Questions
- The figure indicates the enegry level diagram of an atom and the origi...
Text Solution
|
- The Bohr model of atoms
Text Solution
|
- The magnetic moment (mu) of a revolving electron around the nucleus v...
Text Solution
|
- The ground state energy of hydrogen atom is -13.6 eV. What is the pote...
Text Solution
|
- In a Rutherford scattering experiment when a projectile of change Z(1)...
Text Solution
|
- In the Bohr model of a hydrogen atom, the centripetal force is furnish...
Text Solution
|
- In Bohr's model, if the atomic radius of the first orbit is r(0), then...
Text Solution
|
- Given the value of Rydberg constant is 10^(7)m^(-1), the waves number ...
Text Solution
|
- The ratio of the frequencies of the long wavelenght limits of Lyman an...
Text Solution
|
- The wavelength of the first line of Lyman series for hydrogen atom is ...
Text Solution
|
- An electrons of a stationary hydrogen aton passes form the fifth enegr...
Text Solution
|
- Out of the following which one is not a possible energy for a photon t...
Text Solution
|
- The wavelength of the energy emitted when electron come from fourth or...
Text Solution
|
- The diagram shows the energy levels for an electron in a certain atom....
Text Solution
|
- Energy levels A, B, C of a certain atom corresponding to increasing va...
Text Solution
|
- The ionization enegry of the electron in the hydrogen atom in its grou...
Text Solution
|
- The electron in hydrogen atom makes a transition n(1)ton(2) where n1 a...
Text Solution
|
- In Rutherford scattering experiment, what will b ethe correct angle fo...
Text Solution
|
- When an alpha-particle of mass 'm' moving with velocity 'v' bombards o...
Text Solution
|
- If an electron in a hydrogen atom jumps from the 3rd orbit to the 2nd ...
Text Solution
|