A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC AND NUCLEAR PHYSICS
ERRORLESS|Exercise Assertion and Reason|23 VideosATOMIC AND NUCLEAR PHYSICS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Radioactivity)|78 VideosALTERNATING CURRENT
ERRORLESS|Exercise Assertion & Reason|13 VideosCURRENT ELECTRICITY
ERRORLESS|Exercise ASSERTION AND REASON|26 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-ATOMIC AND NUCLEAR PHYSICS-Past Years Questions
- The ratio of kinetic energy to the total energy of an electron in a Bo...
Text Solution
|
- The total energy of an electron in an atom in an orbit is -3.4eV. Its...
Text Solution
|
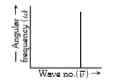
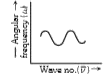
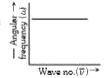
- The graph between wave number (vecv) and angular frequency (omega) is
Text Solution
|
- The mass of a .(3)^(7) Li nucleus is 0.042 u less than the sum of the ...
Text Solution
|
- The binding energies per nucleon for a deuteron and an alpha-particle ...
Text Solution
|
- A certain mass of hydrogen is changes to helium by the process of fusi...
Text Solution
|
- A nucleus .(Z)^(A)X has mass represented by m(A, Z). If m(p) and m(n) ...
Text Solution
|
- In a material medium, when a position meets an electron both the parti...
Text Solution
|
- The dependence of binding energy per nucleon, B(N) on the mass number...
Text Solution
|
- (a) What is nuclear fusion ? Explain with an example. Write the equati...
Text Solution
|
- In an atom bomb, the energy is released because of the.
Text Solution
|
- In any fission the ratio ("mass of fission produts")/("mass of paren...
Text Solution
|
- Atomic weight of boron is 10.81 and it has two isotopes .5 B^10 and .5...
Text Solution
|
- If radius of the .(13)^(27)Al nucleus is taken to be R(AI), then the r...
Text Solution
|
- Two nuclei have their mass numbers in the ratio of 1:3. The ratio of t...
Text Solution
|
- A nucleus ruptures into two nuclear parts, which have their velocity r...
Text Solution
|
- alpha-particle consists of
Text Solution
|
- The decay constant lambda of the radioactive sample is probaility of d...
Text Solution
|
- An archaeologist analyses the wood in a phehistoric structure and find...
Text Solution
|
- The half-life of a radioactive isotope X is 50 years. It decays to ano...
Text Solution
|