A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
KINETIC THEORY OF GASES
ERRORLESS|Exercise ASSERTION AND REASON |16 VideosKINETIC THEORY OF GASES
ERRORLESS|Exercise NCERT BASED QUESTIONS (Pressure and Energy)|31 VideosGRAVITATION
ERRORLESS|Exercise Assertion and Reason |25 VideosMOTION IN ONE DIMENSION
ERRORLESS|Exercise ASSERTION AND REASON|24 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-KINETIC THEORY OF GASES -PAST YEARS QUESTIONS
- According to the kinetic theory of gases, at absolute temperature
Text Solution
|
- Gas at a pressure P(0) in contained as a vessel. If the masses of all ...
Text Solution
|
- At constant volume, temperature is increased. Then
Text Solution
|
- For gas at a temperature T the root-mean-square speed v(rms), the most...
Text Solution
|
- At what temperature will the rms speed of oxygen molecules become just...
Text Solution
|
- Increase in temperature of a gas filled in a container would lead to :
Text Solution
|
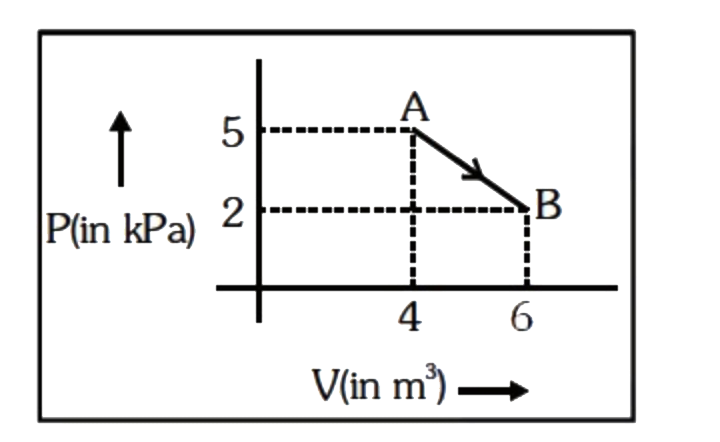
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- The amount of heat energy required to raise the temperature of 1 g of ...
Text Solution
|
- The molar specific heats of an ideal gas at constant pressure and volu...
Text Solution
|
- A gas mixture consists of 2 moles of oxygen and 4 moles of argon at te...
Text Solution
|
- The ratio of the specific heats C(p)/C(v)=gamma in terms of degrees of...
Text Solution
|
- When an ideal monoatomic gas is heated at constant pressure, fraction ...
Text Solution
|
- One mole of an ideal monatomic gas undergoes a process described by th...
Text Solution
|
- In kinetic theory of gases, a molecule of mass m of an ideal gas colli...
Text Solution
|
- The average kinetic energy of H(2) molecules at 300 K is E at the same...
Text Solution
|
- The volume (V) of a monatomic gas varies with its temperature (T) as s...
Text Solution
|
- A cylinder contains hydrogen gas at pressure of 249 kPa and temperatur...
Text Solution
|
- The mean free path for gas with molecular diameter d and number densit...
Text Solution
|
- The average thermal energy for a mono-atomic gas is: (kB is Boltzmann ...
Text Solution
|
- Match Column-I and Column-ll and choose the correct match from the giv...
Text Solution
|