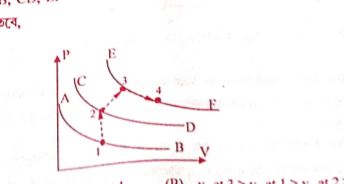
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Certain amount of an ideal gas is taken from its initial state 1 to fi...
Text Solution
|
- A gas take part in two thermal processes in which it is heated from th...
Text Solution
|
- A gas take part in two thermal processes in which it is heated from th...
Text Solution
|
- An ideal gas undergoes isothermal process from some initial state i to...
Text Solution
|
- As shown in figure three paths through which a gas can be taken from t...
Text Solution
|
- A certain gas is taken to the five states representes by dots in the g...
Text Solution
|
- An ideal gas undergoes change in its state from the initial state I to...
Text Solution
|
- An ideal gas undergoes isothermal process from some initial state i to...
Text Solution
|
- An ideal gas undergoes isothermal process from some initial state i to...
Text Solution
|