Read the passage given below and answer the questions that follow :
Oxidaton - reduction reactions are commonly known are redox reactions. They involve transfer of electrons from one species to another . In a spontaneous reaction, energy is released which can be used to do useful work . Thr reaction is split into tow half reaction . Two different containsers are used and a wire is used to drive the electrons form one side to the other and a Voltaic Galvanic cell is created. It is an electrochemical cell that used spnantaneous redox reactions to generate electricity. A salt bridge also connected to the half cells. The reading of the voltmeter gives the cell voltage or cell potential or electromotive force . If `E_("Cell")^(@)` is positive the reaction is spontaneous and if it is negative the reaction is non - spontaneous of a substance by an electric current . One mole of electric charge when passed through a cell will discharge current . One mole of electric charge when paseed through a cell will discharge half a mode of a divalenet metal ion such `Cu^(2+)`. This was first formulated by Faraday in the form of law of electrolysis.
The conductorance of the material is the property of material due to which a materical allows the flow of ions through itself and thus conducts electricy. Conductivity is represented by k and it depend upon nature and concentration of electrolyte temperature etc. A more common term molar conductivity of a solution at a givne concerntration is conductance of the volume of solution containing one mole of electrolyte kept between two electrodes with the unit area of cross - section and distnace of unit lenght. Limiting molar conducitivity of weak electrolytes cannot be obtained graphically.
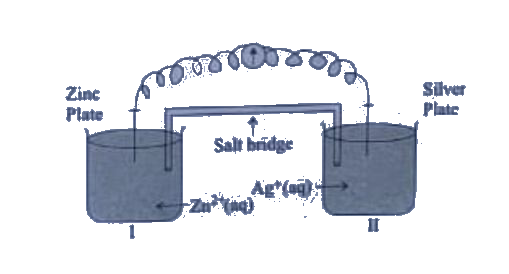
Is silver plate the anode or cathode ?
