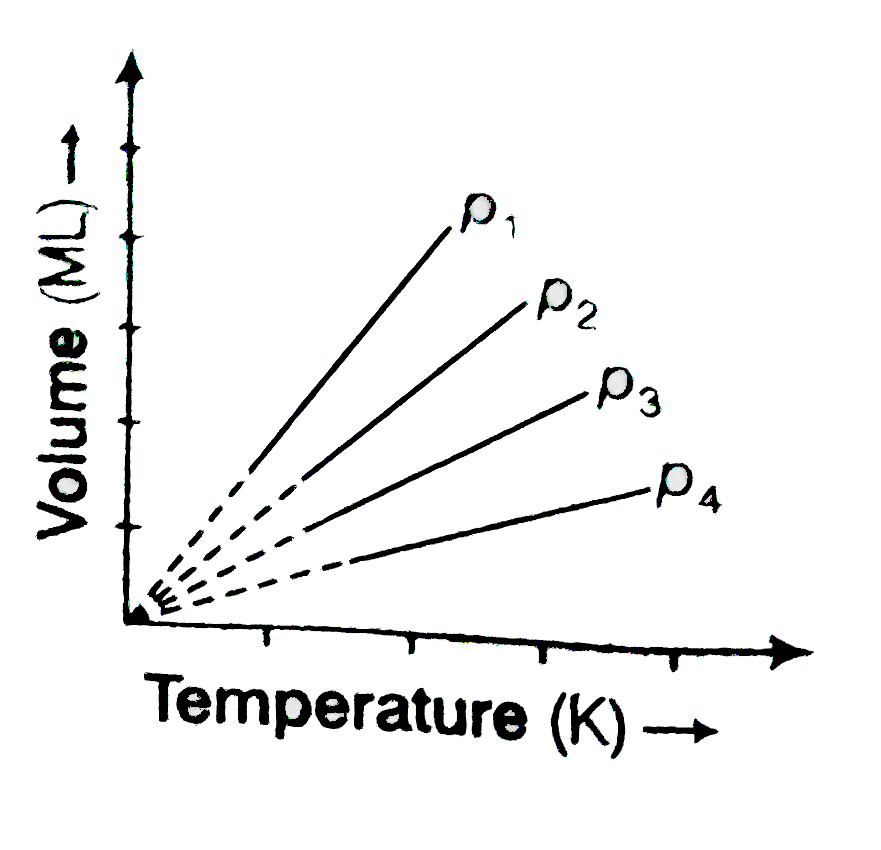
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
ERRORLESS|Exercise NCERT BASED QUESTIONS (KINETIC MOLECULAR THEORY OF GASES AND MOLECULAR COLLISIONS) |12 VideosSTATES OF MATTER
ERRORLESS|Exercise NCERT BASED QUESTIONS (MOLECULAR SPEEDS) |20 VideosSTATES OF MATTER
ERRORLESS|Exercise ASSERTION AND REASON|11 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|10 VideosSTRUCTURE OF ATOM
ERRORLESS|Exercise Assertion & Reason|16 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-STATES OF MATTER -NCERT BASED QUESTIONS (IDEAL GAS EQUATION AND RELATED GAS LAW)
- In order to increase the volume of a gas by 10% , the pressure of the ...
Text Solution
|
- A gas of volume 100 cc, is kept in a vessel at pressure 10^(4)Pa maint...
Text Solution
|
- A plot of volume (V) versus temperature (T) for a gas at constant pres...
Text Solution
|
- Air at sea level is dense. This is a practical application of
Text Solution
|
- Which of the following graph represent Boyle's law ?
Text Solution
|
- Two glass bulbs A and B are connected by a very small tube having a st...
Text Solution
|
- A cylinder of 5 L capacity, filled with air at NTP is connected with a...
Text Solution
|
- Equation for Boyle's law is
Text Solution
|
- A bubble of gas released at the bottom of a lake increases to eight ti...
Text Solution
|
- Pressure remaining the same, the volume of a given mass of an ideal ga...
Text Solution
|
- As the temperature increases, average kinetic energy of molecules incr...
Text Solution
|
- Use of hot air ballons in sports and meteorological observations in a...
Text Solution
|
- The density of O(2) is 16 at STP. At what temperature (in .^@C) its de...
Text Solution
|
- 500mL of NH(3) contains 6.02xx10^(23) molecules at STP. How many mole...
Text Solution
|
- Which of the following mixtures of gases does not obey Dalton’s law of...
Text Solution
|
- Equal weights of two gases of molecular weight 4 and 40 are mixed. The...
Text Solution
|
- Equal weights of methane and hydrogen are mixed in an empty container ...
Text Solution
|
- Which of the following gas mixture is not applicable for Dalton’s law ...
Text Solution
|
- If a mixture of CO and N(2) in equal amount have total 1 atm pressure,...
Text Solution
|
- Steam distillation is based on
Text Solution
|