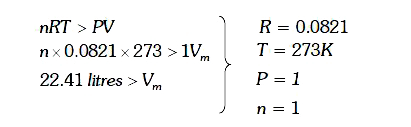A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
ERRORLESS|Exercise NCERT BASED QUESTIONS (CRITICAL STATE AND LIQUEFACTION OF GASES) |16 VideosSTATES OF MATTER
ERRORLESS|Exercise NCERT BASED QUESTIONS (VISCOSITY & SURFACE TENSION) |11 VideosSTATES OF MATTER
ERRORLESS|Exercise NCERT BASED QUESTIONS (MOLECULAR SPEEDS) |20 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|10 VideosSTRUCTURE OF ATOM
ERRORLESS|Exercise Assertion & Reason|16 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-STATES OF MATTER -NCERT BASED QUESTIONS (REAL GASES AND VAN DER WAALS EQUATION)
- The term that corrects for the attractive forces present in a real gas...
Text Solution
|
- The compressibility factor of a gas is defined as z = PV //RT . The co...
Text Solution
|
- In van der Waals equation of state for a non-ideal gas , the term that...
Text Solution
|
- Van der Waal's equation of state is obeyed by real gases. For n moles ...
Text Solution
|
- Any gas shows maximum deviation from ideal gas behaviour at
Text Solution
|
- van der Waal's equation reduces itself to the ideal gas equation at
Text Solution
|
- A gas deviated from ideal behaviour at a high pressure because its mol...
Text Solution
|
- A gas is said to behave like an ideal gas when the relation (pV)/(T)= ...
Text Solution
|
- A real gas most closely approaches the behaviour of an ideal gas at
Text Solution
|
- At high temperature and low pressure the van der Waals equation is red...
Text Solution
|
- Pressure exerted by 1 mole of methane in a 0.25 litre container at 300...
Text Solution
|
- What is the pressure of 2 mole of NH(3) at 27^(@)C when its volume is ...
Text Solution
|
- The compressibility of a gas is less than unity at STP .
Text Solution
|
- The temperature at which the second virial coefficient of a real gas i...
Text Solution
|
- van der Waal's constant 'a' and 'b' are related with respectively
Text Solution
|
- The vander waal's constant "a" for gases P,Q,R and S are 4.17 , 359 ....
Text Solution
|
- The temperature at which a real gas obeys the ideal gas laws over a wi...
Text Solution
|
- The units of constants a in van der Waals' equations is
Text Solution
|
- For real gases, van der Waals' equation is written as (P+(an^(2))/(V...
Text Solution
|
- In the following compressibility factor (Z ) vs pressure graph at K 30...
Text Solution
|