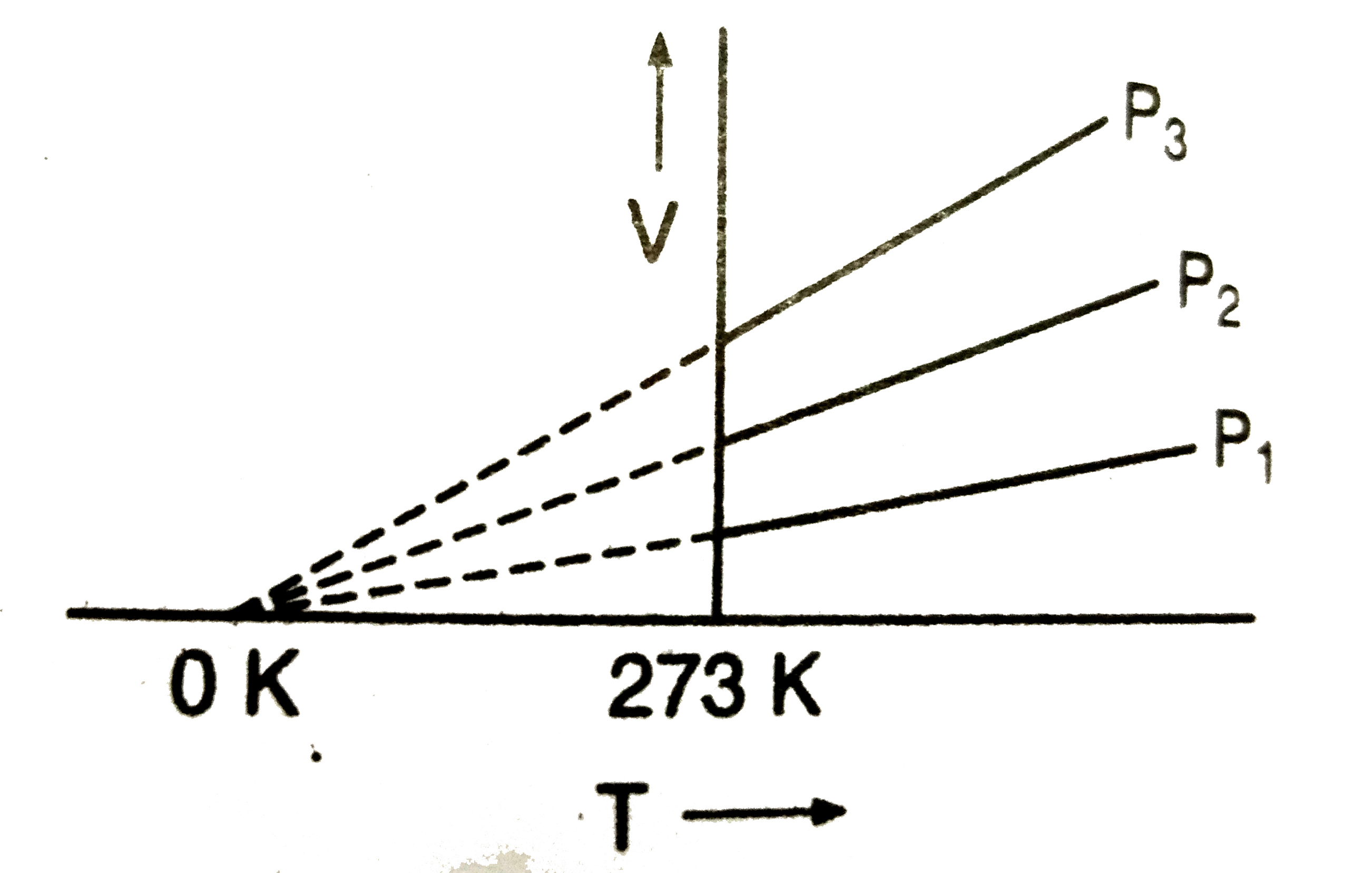
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER
ERRORLESS|Exercise ASSERTION AND REASON|11 VideosSTATES OF MATTER
ERRORLESS|Exercise NCERT BASED QUESTIONS (VISCOSITY & SURFACE TENSION) |11 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|10 VideosSTRUCTURE OF ATOM
ERRORLESS|Exercise Assertion & Reason|16 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-STATES OF MATTER -PAST YEAR QUESTIONS
- Pressure of a mixture of 4 g of O(2) and 2 g H(2) confined in a bulb o...
Text Solution
|
- The density of a gas at 27^(@)C and 1 atm is d. Pressure remaining con...
Text Solution
|
- The volume-temperature graphs of a given mass of an ideal gas at const...
Text Solution
|
- A certain gas takes three times as long to effuse out as helium. Its m...
Text Solution
|
- Equal masses of H(2), O(2) and methane have been taken in a container ...
Text Solution
|
- Two gases A and B having the same volume diffuse through a porous part...
Text Solution
|
- 50 mL of each gas A and of gas B takes 150 and 200 seconds respectivel...
Text Solution
|
- Equal moles of hydrogen and oxygen gases are placed in a container wit...
Text Solution
|
- A 20 litre container at 400 K contains CO(2) (g) at pressure 0.4 atm a...
Text Solution
|
- At STP, 0.50 mole H(2) gas and 1.0 mole He gas
Text Solution
|
- Absolute zero is defined as the temperture
Text Solution
|
- The ratio among most probable velocity, mean velocity and root mean ve...
Text Solution
|
- The temperature of the gas is raised from 27^(@)C to 927^(@)C, the roo...
Text Solution
|
- Dominance of strong repulsive forces among the molecules of the gas (Z...
Text Solution
|
- Consider the following liquid-vapour equilibrium. "Liquid" hArr "Vap...
Text Solution
|
- Maximum deviation from ideal gas is expected from
Text Solution
|
- The correction factor 'a' to the ideal gas equation corresponds to
Text Solution
|
- A gas at 350 K and 15 bar has molar volume 20 percent smaller than tha...
Text Solution
|
- An ideal gas cannot be liquified because
Text Solution
|
- An ideal gas obeying the kinetic theory of gases can be liquefied if
Text Solution
|