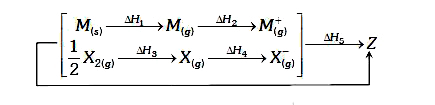A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS AND THERMOCHEMISTRY
ERRORLESS|Exercise NCERT Based Questions (First Law of Thermodynamics and Hess Law)|55 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
ERRORLESS|Exercise NCERT Based Questions (IInd & IIIrd Law of Thermodynamics and Entropy)|41 VideosTHE S-BLOCK ELEMENTS
ERRORLESS|Exercise Assertion & Reason|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THERMODYNAMICS AND THERMOCHEMISTRY-(Assertion & Reason)
- Consider the Born- Haber cycle for the formation of an ionic compound ...
Text Solution
|
- Assertion: The enthalpy of formation of gaseous oxygen molecules at 29...
Text Solution
|
- Assertion: We feel cold on touching the ice. Reason: Ice is a solid ...
Text Solution
|
- Assertion: Entropy of ice is less than water. Reason: Ice has cage-lik...
Text Solution
|
- Assertion: The heat absorbed during the isothermal expansion of an ide...
Text Solution
|
- Assertion:Absolute values of intenal energy of substances cannot be d...
Text Solution
|
- Assertion: Mass and volume are extensive properties. Reason: Mass/vo...
Text Solution
|
- Assertion : Molar entropy of vaporization of water is different from e...
Text Solution
|
- Assertion:The increase in internal energy(DeltaE)for the vaporiation o...
Text Solution
|
- Assertion: The increase in internal energy (DeltaE) for the vaporisati...
Text Solution
|
- Assertion: DeltaH and DeltaE are almost the same for the reaction. N(2...
Text Solution
|
- Assertion:The enthalpies of neutralised of strong acids and strong bas...
Text Solution
|
- Assertion : Zeroth law can also be termed as law of thermal equilibriu...
Text Solution
|
- Assertion : For an isothermal reversible process Q = - w i.e., work do...
Text Solution
|
- Assertion :- Water in liquid state is more stable than ice at room tem...
Text Solution
|
- Assertion : In an isolated system the entropy increases. Reason : Th...
Text Solution
|
- Assertion : For the combustion of methane, DeltaE gt DeltaH. Reason :...
Text Solution
|
- (A) For reaction 2NH(3)(g)toN(2)(g)+3H(2)(g)," "DeltaHgtDeltaE (R...
Text Solution
|
- Assertion:The change in entropy during melting of ice is negligible in...
Text Solution
|