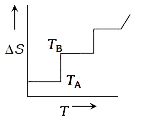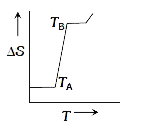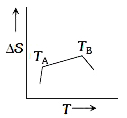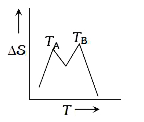A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS AND THERMOCHEMISTRY
ERRORLESS|Exercise NCERT Based Questions (Heat of Reaction)|67 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
ERRORLESS|Exercise NCERT Based Questions (Bond Energy)|15 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
ERRORLESS|Exercise NCERT Based Questions (First Law of Thermodynamics and Hess Law)|55 VideosTHE S-BLOCK ELEMENTS
ERRORLESS|Exercise Assertion & Reason|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THERMODYNAMICS AND THERMOCHEMISTRY-NCERT Based Questions (IInd & IIIrd Law of Thermodynamics and Entropy)
- In which of the following entropy decreases
Text Solution
|
- For conversion C (graphite)rarrC (diamond) the DeltaS is
Text Solution
|
- If for a given substance, melting point is T(B) and freezing point is ...
Text Solution
|
- Which of the following would be expected to have the largest antropy p...
Text Solution
|
- Mixing of non-reacting gases is generally accompanied by
Text Solution
|
- An irreversible process occurring isothermally in an isolated system l...
Text Solution
|
- For chemical reactions, the calculation of change in entropy is normal...
Text Solution
|
- For which of the following processes is DeltaS negative?
Text Solution
|
- A container has hydrogen and oxygen mixture in ratio of 4 : 1 by weigh...
Text Solution
|
- For the gas phase reactions PCl(5)(g) hArr PCl(3)(g) + Cl(2)(g) Whi...
Text Solution
|
- Considering entropy (S) as a thermodynamics parameter, the criterion f...
Text Solution
|
- Entropy changesh for the process, H(2)O(l) to H(2)O at normal pressure...
Text Solution
|
- For an exothermic reaction, which is true
Text Solution
|
- The entropy change can be calculated by using the expression DeltaS-(q...
Text Solution
|
- A : For an isolated system Delta G = - TDeltaS("total") R: For an i...
Text Solution
|
- For stable equilibrium, which of the following is/are true ?
Text Solution
|
- A concentrated solution of copper sulphate, which is dark blue in c...
Text Solution
|
- As isolated box, equally partitioned contains two ideal gases A and B ...
Text Solution
|
- For a process to occurs spontaneously
Text Solution
|
- The latent heat of melting of ice at 0^(@)C is 6 kJ "mol"^(-1) . The e...
Text Solution
|