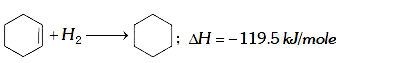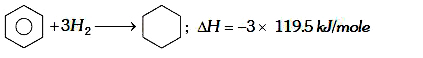A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-THERMODYNAMICS AND THERMOCHEMISTRY-PAST Years Questions
- If the end energies of H-H, Br-Br and H-Br are 433, 192 and 364 kJ mol...
Text Solution
|
- Enthalpy change for the reaction, 4H((g))rarr 2H(2(g)) is -869.6 kJ ...
Text Solution
|
- The enthalpy of hydrogenation of cyclohexene is -119.5kJ mol^(-1). If ...
Text Solution
|
- The values of DeltaH and DeltaS for the reaction, C("graphite")+CO(2...
Text Solution
|
- Which of the following pairs of a chemical reaction is certaion to res...
Text Solution
|
- The enthalpy and entropy change for the reaction Br(2)(l) + Cl(2)(g)...
Text Solution
|
- The standard enthalpy or the decomposition of N2 O5 to NO2 is 58.04 kJ...
Text Solution
|
- Standard enthalpy and standard entropy changes for the oxidation of am...
Text Solution
|
- For vaporization of water at 1 atmospheric pressure, the values of Del...
Text Solution
|
- Using the Gibbs energy change, Delta G^(@)=+ 63.3 kJ, for the followin...
Text Solution
|
- Which of the following statements is correct for a reversible process ...
Text Solution
|
- The correct thermodynamic conditions for the spontaneous reaction at a...
Text Solution
|
- Which of the following is true fo the reacion H(2)O(l)hArr H(2)O(g) at...
Text Solution
|
- For a spontaneous process the correct statement is -
Text Solution
|
- For the equilibrium H(2)O(l) iff H(2)O(g) at 1 atm and 298 K
Text Solution
|
- Match List I ( Equations) with List II (Type of processes) and select...
Text Solution
|
- For a given reaction, Delta H = 35.5 kJ mol^(-1) and Delta S = 83.6 JK...
Text Solution
|
- Which of the following is correct option for the free expansion of an ...
Text Solution
|
- For the reaction, 2Cl(g) rarr Cl2(g), the correct option is:
Text Solution
|
- For a reaction AtoB, enthalpy of reaction is -4.2kJmol^(-1) and enthal...
Text Solution
|