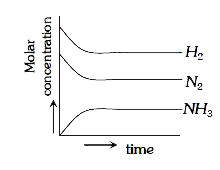
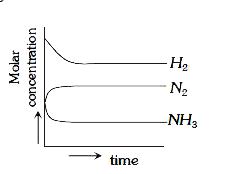
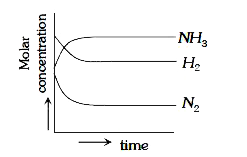
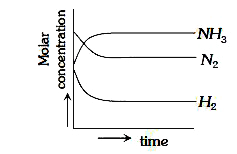A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
ERRORLESS|Exercise NCERT BASED QUESTION (Law of Mass Action)|7 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise NCERT BASED QUESTION (Law of Equilibrium and Equilibrium Constant)|65 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise Assertion & Reason |17 VideosCLASSFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
ERRORLESS|Exercise Assertion & Reason |14 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL EQUILIBRIUM-NCERT BASED QUESTION (Equilibrium State)
- In the given reaction N2 + O2 hArr 2NO, equilibrium means that
Text Solution
|
- In chemical reaction A hArr B, the system will be know in equilibrium...
Text Solution
|
- Which statement characterize a chemical system at equilibrium ? (P)t...
Text Solution
|
- The number of gram molecules of a substance present in unit volume is ...
Text Solution
|
- In a chemical reaction, equilibrium is said to have been established w...
Text Solution
|
- For the synthesis of ammonia by the reaction N(2)+3H(2)hArr2NH(3) in t...
Text Solution
|
- Which of the following is not a general characteristic of equilibrium ...
Text Solution
|
- CH(3)CHOO((1))+C(2)H(5)OH((1))hArrCH(3)COOC(2)H(5(1))+H(2)O((1)) In th...
Text Solution
|