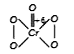
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDOX REACTIONS
ERRORLESS|Exercise NCERT BASED QUESTION (Oxidizing and Reducing Agent)|43 VideosREDOX REACTIONS
ERRORLESS|Exercise NCERT BASED QUESTION (Auto Oxidation and Disproportionation)|11 VideosPURIFICATION, CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
ERRORLESS|Exercise ASSERTION & REASON|9 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-REDOX REACTIONS-ASSERTION & REASON
- In acidic medium, H(2)O(2) changes Cr(2)O(7)^(-2) to CrO(5) which has ...
Text Solution
|
- Assertion (A): SO(2) and Cl(2) are both bleaching agents. Reason (R ...
Text Solution
|
- Assertion(A): Fluorine exists only in -1 oxidation state. Reason(R):...
Text Solution
|
- Assertion: Stannous chloride is a powerful oxidising agent which oxidi...
Text Solution
|
- Assertion: HClO(4) is a stronger acid than HClO(3). Reason: Oxidatio...
Text Solution
|
- Assertion(A) : The oxidation numbers are artificial, they are useful a...
Text Solution
|
- Assertion :- Equivalent weight of NH(3) in the reaction N(2) rarrNH(3)...
Text Solution
|