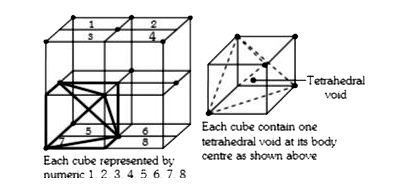
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-SOLID STATE-ASSERTION & REASON
- Calculate the number of tetrahedral voids in the unit cell of a face-c...
Text Solution
|
- Assertion : Diamond is a precious stone Reason : carbon atomic are ...
Text Solution
|
- Assertion: In a crystal, the size of the cation is larger in a tetrahe...
Text Solution
|
- Assertion : Crystalline solids have short range order. Reason : Amor...
Text Solution
|
- Assertion: Quartz glass is crystalline solid and quartz is an amorphou...
Text Solution
|
- Assertion: The presence of a large number of Schottky defects in NaCl ...
Text Solution
|
- Assertion: Anion vacancies in alkali halides are produced by heating t...
Text Solution
|
- Assertion : On heating ferromagnetic or ferromagnetic substance , the...
Text Solution
|
- Assertion :Lead zirconate is a piezoelectric crystal Reason : Lead ...
Text Solution
|
- Assertion. No compound has both Schottky and Frenkel defects. Reaso...
Text Solution
|
- Assertion . Graphite is an example to hexogonal crystal system. Reas...
Text Solution
|
- Ammong the following, the element having highest ionization enthalpy a...
Text Solution
|