A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-SOLID STATE-PAST YEARS QUESTIONS
- A compound is compound by cation C and anion A. The anions hexagonal c...
Text Solution
|
- CsBr crystallises in a body centered cubic lattice. The unit cell leng...
Text Solution
|
- Potassium has a bcc structure with nearest neighour distance 4.52 Åits...
Text Solution
|
- A metal has a fcc lattice.The edge length of the unit cell is 404 pm ,...
Text Solution
|
- An element (atomic mass = 100 g//mol) having bcc structure has unit ce...
Text Solution
|
- Lithium has a bcc structure. Its density is 530 kg m^(-3) and its atom...
Text Solution
|
- The pyknometric density of sodium chloride crystal is 2.165xx10^(3)kg ...
Text Solution
|
- The radii of Na^(+) and Cl^(-) ions are 95 pm and 181 pm respectively....
Text Solution
|
- A solid compound XY has NaCl structure. If the radius of the cation is...
Text Solution
|
- If the pressure on a NaCI structure in increases , then its coordinati...
Text Solution
|
- The ionic radii of A^(+) and B^(-) ions are 0.98 xx 10^(-10) and 1.81 ...
Text Solution
|
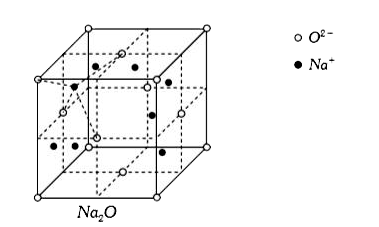
- What is the co-ordination number of sodium in Na(2)O ?
Text Solution
|
- In calcium, fluoride having the florite structures. The coordination n...
Text Solution
|
- The correct statement regarding defects in crystalling solids.
Text Solution
|
- The appearance of colour in solid alkali metal halides is generally du...
Text Solution
|
- If NaCl is doped with 10^(-4)mol%of SrCl(2) the concentration of cati...
Text Solution
|
- Which is the incorrect statement
Text Solution
|
- An element has a body centered cubic (bcc) structure with a cell edge ...
Text Solution
|
- Right option for the number oof tetrahedral and octahedral voids in he...
Text Solution
|
- The correct option for the number of body centred unit cells in all 14...
Text Solution
|