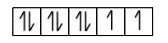
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Compounds of Transitional Elements)|163 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Past Years Questions |55 VideosSURFACE CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 VideosTHE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise ASSERTION & REASON|22 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THE d-AND f- BLOCK ELEMENTS -Assertion and Reason
- Magnetic moment 2.83 BM is given by which of the following ions? "At...
Text Solution
|
- Assertion : Cuprous ion (Cu^(+)) has unpaired electrons while cupric i...
Text Solution
|
- Cabalt-60 is useful in cancer therapy. Cabalt-60 is source of Y-radi...
Text Solution
|
- Assertion: Transitio metals show variable valence. Reason : Due to a...
Text Solution
|
- Assertion : The aqueous solution of FeCl(3) is basic in nature . Rea...
Text Solution
|
- Assertion : AgC1 dissolves in NH(4) OH solution. Reason: Due to form...
Text Solution
|
- Assertion : Pure iron is not used for making tools and machines. Rea...
Text Solution
|
- Assertion : Solution of Na(2) CrO(4) in water is intensely electrons. ...
Text Solution
|
- Assertion (A) : Cu gets readily corroded in acidic aqueous solution. ...
Text Solution
|
- Assertion : The free gases Cr atom has six unpaired electrons. Half-...
Text Solution
|
- Assertion : Mercury vapour is shining silvery in appearance. Reason ...
Text Solution
|
- Statement-I : Extraction of iron metal from iron oxide ore is carried ...
Text Solution
|