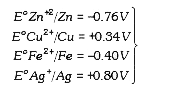A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Past Years Questions |55 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 VideosSURFACE CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 VideosTHE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise ASSERTION & REASON|22 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THE d-AND f- BLOCK ELEMENTS -NCERT BASED QUESTIONS (Compounds of Transitional Elements)
- The protection of steel by chrome plating is due to
Text Solution
|
- KI and CuSO4 solution when mixed give .
Text Solution
|
- Copper displaces Which of the metal from their salt solutions?
Text Solution
|
- From a solution of CuSO4 the metal used to recover copper is
Text Solution
|
- If excess of NH(4)OH is added to CuSO(4) solution, it forms blue colou...
Text Solution
|
- When CuSO4 solution is added to K4[Fe(CN)6], the formula of the produ...
Text Solution
|
- When metallic copper comes in contact with mositure, a green powdery/p...
Text Solution
|
- Colourless solutions of the following four salts are placed separately...
Text Solution
|
- The metal which can be used to obtain metallic Cu from aqueou...
Text Solution
|
- Identify the statement which is not correct regarding CuSO4?
Text Solution
|
- On adding excess of NH(3) solution to CuSO(4) solution, the dark blue ...
Text Solution
|
- A blue colouration is not obtained when
Text Solution
|
- What is the effect of shaking dil. H(2)SO(4) with small quantity of an...
Text Solution
|
- Which among the following alloys is used in making instruments for el...
Text Solution
|
- When CuSO(4) is hydrated, then it becomes
Text Solution
|
- Copper sulphate solution reacts with KCN to give (a) Cu(CN)(2) (b)...
Text Solution
|
- Why does AgNO(3) produced a black stain on the skin?
Text Solution
|
- Silver nitrate is supplied in coloured bottles because it is
Text Solution
|
- Which of the following metal hydroxides does not dissolve in sodium hy...
Text Solution
|
- Silver nitrate solution is used to study
Text Solution
|