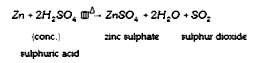A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Past Years Questions |55 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 VideosSURFACE CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 VideosTHE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise ASSERTION & REASON|22 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THE d-AND f- BLOCK ELEMENTS -NCERT BASED QUESTIONS (Compounds of Transitional Elements)
- AgCl dissolved in excess of NH(3), KCN and Na(2)S(2)O(3) solutions the...
Text Solution
|
- A copper coin is completely covered with a gold film and is placed in ...
Text Solution
|
- Name the reagent that is used in leaching of gold .
Text Solution
|
- A compound of Zinc which is white in cold state and yellow in hot stat...
Text Solution
|
- White vitriol is
Text Solution
|
- In the metallurgy of Zn the Zn dust obtained from roasting and reducti...
Text Solution
|
- Zinc when reacted with excess of NaOH gives
Text Solution
|
- Lucas reagent is ,
Text Solution
|
- Zinc reacts with hot and concetrated H(2)SO(4) to give
Text Solution
|
- Reduction of zinc with cold and very dilute nitric acid yields
Text Solution
|
- What happens when aluminium and zinc salts react with an excess of NaO...
Text Solution
|
- When calomel react with NH(4)OH solution the compound formed is
Text Solution
|
- The formula of corrosive sublimate is
Text Solution
|
- The main product obtained when a solution of sodium carbonate reacts w...
Text Solution
|
- The gas produced on heating MnO(2) with conc. HCl is -
Text Solution
|
- The number of moles of KMnO(4) required to oxidize one equivalent of K...
Text Solution
|
- The charge required for the reduction of 1 mole of Cr(2)O(7)^(2-) ions...
Text Solution
|
- In alkaline medium, the reaction of hydrogen peroxide with potassium p...
Text Solution
|
- The major product formed in the oxidation of acetylene with alkaline K...
Text Solution
|
- Upon heating with acidic KMnO4 an organic compound produces hexan – 1,...
Text Solution
|