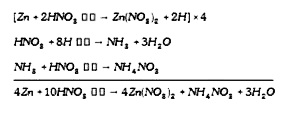A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise Assertion and Reason |11 VideosTHE d-AND f- BLOCK ELEMENTS
ERRORLESS|Exercise NCERT BASED QUESTIONS (Compounds of Transitional Elements)|163 VideosSURFACE CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 VideosTHE P -BLOCK ELEMENTS (NITROGEN, OXYGEN, HALOGEN AND NOBLE FAMILY)
ERRORLESS|Exercise ASSERTION & REASON|22 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-THE d-AND f- BLOCK ELEMENTS -Past Years Questions
- Which of the statements is not true ?
Text Solution
|
- Which of the following does not give oxygen on heating ?
Text Solution
|
- KMnO4 can be prepared fromK2MnO4 as per the reaction: The reaction ...
Text Solution
|
- The reaction of aqueous KMnO(4) with H(2)O(2) i acidic conditions give...
Text Solution
|
- F2 is the formed by reacting K2MnF6 with
Text Solution
|
- Assuming complete ionization, same moles of which of the following com...
Text Solution
|
- To protect iron against corrosion, the most suitable metal plating on ...
Text Solution
|
- When copper is heated with conc.HNO(3) it produces
Text Solution
|
- Silver obtained from argentiferrous lead containing lead impurity is p...
Text Solution
|
- Which of the following metals is obtained by leaching out process usin...
Text Solution
|
- Which of the following does not react with AgCl?
Text Solution
|
- Zn gives H(2) gas with H(2)SO(4) and HCl but not with HNO(3) because
Text Solution
|
- The compound insoluble in water is
Text Solution
|
- Name the gas that can readily decolourise acidified KMnO(4) solution:
Text Solution
|
- The magnitude and permanganate ions are tetrahedral due to
Text Solution
|
- The calculated spin only magnetic moment of Cr^2+ ion is:
Text Solution
|
- Identify the incorrect statement
Text Solution
|
- Urea reacts with water to form A which will decompose to form B. B whe...
Text Solution
|
- Zr(Z=40) and Hf(Z=72) have similar atomic and ionic radii because of:
Text Solution
|
- The incorrect statement among the following is
Text Solution
|