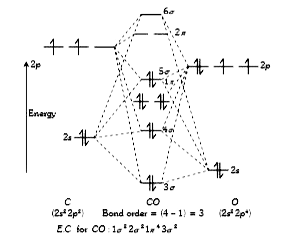
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
ERRORLESS|Exercise PAST YEARS QUESTIONS|60 VideosSTRUCTURE OF ATOM
ERRORLESS|Exercise Assertion & Reason|16 VideosSTRUCTURE OF ATOM
ERRORLESS|Exercise NCERT BASED QUESTIONS (Uncertainty Principle and Schrodinger Wave Equation) |13 VideosSTATES OF MATTER
ERRORLESS|Exercise ASSERTION AND REASON|11 VideosTHE P-BLOCK ELEMENTS (BORON AND CARBON FAMILY)
ERRORLESS|Exercise Assertion & Reason|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-STRUCTURE OF ATOM-NCERT BASED QUESTIONS (Quantum Number, Electronic Configuration and Shape of Orbitals)
- The maximum number of electrons in an atom with l=2 and n=3 is
Text Solution
|
- Two electrons have the following quantum numbers : P=3, 2, -2, +1/2 ...
Text Solution
|
- what is the ground state electronic configuration of a (27) CO atom i...
Text Solution
|
- The element with quantum numbers n=2, l=1, m=1, s=-1/2 has the followi...
Text Solution
|
- Electrons will first enter into which set of quantum numbers-n = 5, l ...
Text Solution
|
- For sodium atom the number of electrons with m=0 will be
Text Solution
|
- If n = 6, the correct sequence for filling of electrons will be.
Text Solution
|
- Identify the CORRECT statement
Text Solution
|
- The explanation for the presence of three unpaired electrons in the ni...
Text Solution
|
- Pairing of electron in an degenerate orbitals takes place only when th...
Text Solution
|
- Which of the following sequence is correct as per Aufbau principle ?
Text Solution
|
- Electron enters the sub-shell for which (n+l) value is minimum. This i...
Text Solution
|
- Following Hund's rule which element contains six unpaired electron
Text Solution
|
- The orbital diagram in which the Aufbau's principle is violated is
Text Solution
|
- Which of the following principles/limits the maximum number of electro...
Text Solution
|
- The correct ground state electronic configuration of chromium atom is ...
Text Solution
|
- An element forms diatomic molecule with a triple bond. The configurati...
Text Solution
|
- Electronic configuration of deuterium atom is
Text Solution
|
- Electronic configurations of Cu ( Z = 29) is
Text Solution
|
- Krypton (At. No 36) has the electronic configuraiton [Ar] 4s^(2)3d^(10...
Text Solution
|