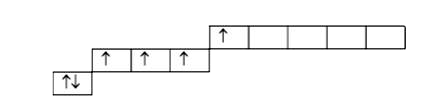
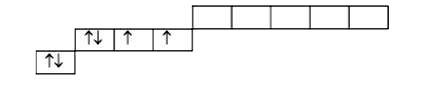
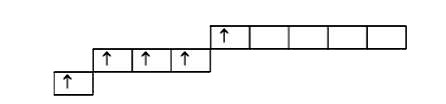
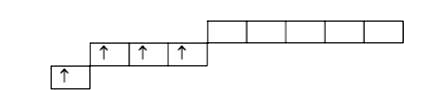
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
ERRORLESS|Exercise PAST YEARS QUESTIONS|60 VideosSTRUCTURE OF ATOM
ERRORLESS|Exercise Assertion & Reason|16 VideosSTRUCTURE OF ATOM
ERRORLESS|Exercise NCERT BASED QUESTIONS (Uncertainty Principle and Schrodinger Wave Equation) |13 VideosSTATES OF MATTER
ERRORLESS|Exercise ASSERTION AND REASON|11 VideosTHE P-BLOCK ELEMENTS (BORON AND CARBON FAMILY)
ERRORLESS|Exercise Assertion & Reason|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-STRUCTURE OF ATOM-NCERT BASED QUESTIONS (Quantum Number, Electronic Configuration and Shape of Orbitals)
- Correct configuration of Fe^"+3" [26] is
Text Solution
|
- What is the electronic configuration of Cu^(2+)(Z=29) of least positio...
Text Solution
|
- Which one is the ground state
Text Solution
|
- Which of the following electronic configurations is not possible
Text Solution
|
- The outer electronic structure of 3s^2 3p^5 is possessed by
Text Solution
|
- Which of the following electronic configuration is not possible accord...
Text Solution
|
- Total number of unpaired electrons in an atom of atomic number 29 is
Text Solution
|
- v41newFlow
Text Solution
|
- Which of the following ions has the maximum number of unpaired d-elect...
Text Solution
|
- Which of the following options does not represent ground state electr...
Text Solution
|
- The pair of ions having same electronic configuration is.
Text Solution
|
- Correct energy value order is
Text Solution
|
- Out of the following electronic arrangements for outer electronic conf...
Text Solution
|
- Probability of finding an electrons at the nodal surface is
Text Solution
|
- Maximum number of electrons in a sub-shell of an atom is determined by...
Text Solution
|
- Which orbital give an electron the greatest probability of being found...
Text Solution
|
- The valency of the element having atomic number 9 is
Text Solution
|
- A completely filled d -orbital (d^(10)) is
Text Solution
|
- Number of unpaired electrons in Mn^(4+) is
Text Solution
|
- Number of nodal centres for 2s orbital
Text Solution
|