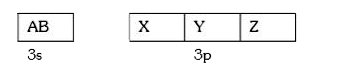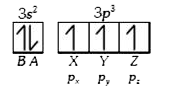A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
ERRORLESS|Exercise Assertion & Reason|16 VideosSTRUCTURE OF ATOM
ERRORLESS|Exercise NCERT BASED QUESTIONS (Quantum Number, Electronic Configuration and Shape of Orbitals) |95 VideosSTATES OF MATTER
ERRORLESS|Exercise ASSERTION AND REASON|11 VideosTHE P-BLOCK ELEMENTS (BORON AND CARBON FAMILY)
ERRORLESS|Exercise Assertion & Reason|10 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-STRUCTURE OF ATOM-PAST YEARS QUESTIONS
- What is the maximum number of orbitals that can be identified with the...
Text Solution
|
- Two electrons occupying the same orbital are distinguished by:
Text Solution
|
- Five valence electrons of ""(15)P are labelled as If the spin qua...
Text Solution
|
- The statements. (i) In filling a group of orbitals of equal energy, ...
Text Solution
|
- Which of the following explains the sequence of filling the electrons ...
Text Solution
|
- Which one is the correct outer configuration of chromium.
Text Solution
|
- Elements up to atomic number 103 have been synthesized and studied. If...
Text Solution
|
- Which one is the electronic configuration of Fe^(+2)
Text Solution
|
- The electronic configuration of gadolinium (atomic number 64) is:
Text Solution
|
- Which one pair of atoms or ions will have same configuration ?
Text Solution
|
- The configuration 1s^(2)2s^(2)2p^(5)3s^(1) shown
Text Solution
|
- Number of unpaired electrons in 1s^(2),2s^(2)2p^(3) is
Text Solution
|
- Which of the following is not correct for electron distribution in the...
Text Solution
|
- What is the orbital angular momentum for an electron in d orbital ?
Text Solution
|
- Which of the following has maximum energy ?
Text Solution
|
- How many delectrons can fit in the orbital for which n = 3 and l = 1?
Text Solution
|
- The orbital angular momentum of a p-electron is given as :
Text Solution
|
- For the energy level is an atom which one of the following statement i...
Text Solution
|
- The number of d-electrons in Fe^(2+)(Z=26) is not equal to the number...
Text Solution
|
- Which of the following pairs of d-orbitals will hare electron density ...
Text Solution
|