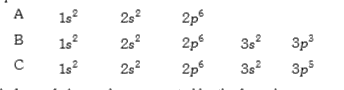A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Co-ordinate or Dative Bonding)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (VSEPR Theory)|46 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise Assertion & Reason |17 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-NCERT BASED QUESTIONS (Covalent Bonding)
- Which one in the following contains ionic as well as covalent bond
Text Solution
|
- Which of the following bonds has the most polar character ?
Text Solution
|
- The electronic configuration ofhte elements. A, B and C are given belo...
Text Solution
|
- The electronic configuration of the elements A, B and C are given belo...
Text Solution
|
- The electronic configuration of the elements A, B and C are given belo...
Text Solution
|
- The electronic configuration of the elements A, B and C are given belo...
Text Solution
|
- The electronic configuration of the outer most shell of the most elect...
Text Solution
|
- Among CaH2, NH3, NaH and B2H6 which are covalent hydrides?
Text Solution
|
- Which of the following substances has giant covalent structure
Text Solution
|
- Strongest bond is
Text Solution
|
- Which of the following occurs, when two hydrogen atoms bond with each ...
Text Solution
|
- Which is the most covalent?
Text Solution
|
- Among the following the maximum covalent character is shown by the com...
Text Solution
|
- The correct sequence of increasing covalent character is represented b...
Text Solution
|
- Boron form covalent compound due to
Text Solution
|
- Covalent compounds have low melting points because
Text Solution
|
- Among the species CO(2),CH(3)COO',CO,CO(3)^(2-)HCHO which has the veak...
Text Solution
|
- The covalent bond length is the shortest in which of the following bon...
Text Solution
|
- Which of the following bonds require the largest amount of bond energy...
Text Solution
|
- Which can described as a molecule with residual bonding capacity
Text Solution
|