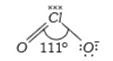
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Hybridisation)|67 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Dipole Moment)|25 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Co-ordinate or Dative Bonding)|10 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-NCERT BASED QUESTIONS (VSEPR Theory)
- The shape of "XeOF"(2) on the basis of VSEPR theory is
Text Solution
|
- In XeF(6), oxidation state and of hybridisation of Xe and shape of the...
Text Solution
|
- Which of the following species is non-linear ?
Text Solution
|
- According to VSEPR theory, the geometry of the molecule OF2 would be
Text Solution
|
- Incorrect matching amongest the following is (i) Linear -H(2)O,SO(2)...
Text Solution
|
- Among the following molecules : SO(2),SF(4) ,CIF(3) ,BrF(5) , and XeF(...
Text Solution
|
- The number of lone pair(s) of electrons on the central atom in [BrF4]^...
Text Solution
|
- The shape around the central form in ClF(4)^(+) is
Text Solution
|
- Fluorine reacts with dilute NaOH and forms a gaseous product A. The bo...
Text Solution
|
- The geometry of ClO(3)^(-) ion according to valence shell electron pai...
Text Solution
|
- In NO(3)^(-) ion, the number of bond pairs and lone pairs of electrons...
Text Solution
|
- In which of the following molecule/ion all the bonds are not equal?
Text Solution
|
- How many electrons pairs are present in valence shell of oxygen in wat...
Text Solution
|
- According to VSEPR theory, the most probable shape of the molecule hav...
Text Solution
|
- Which of the following is the correct reducing order of bondangle
Text Solution
|
- True order of bond angle is
Text Solution
|
- Which one of the following molecules has the smallest bond angle ?
Text Solution
|
- Out of N(2) O , SO(2), I(3)^(+), I(3)^(-), H(2)O, NO(2)^(-) and N(3)^...
Text Solution
|
- Correct order of bond angles for the following is
Text Solution
|
- For overset(Theta)NH(2) the best three-dimensional view is .
Text Solution
|