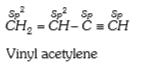A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Dipole Moment)|25 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Polarisation and Fajan.s Rule)|14 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (VSEPR Theory)|46 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-NCERT BASED QUESTIONS (Hybridisation)
- All carbon atoms are sp^(2) hybridised in
Text Solution
|
- Which of the following pairs is isostractural (i.e having the same sha...
Text Solution
|
- State of hybridization of carbon atoms in vinyl acetylene is/are
Text Solution
|
- The hybridisations of atomic orbitals of nitrogen in NO2, NO3^(-) and ...
Text Solution
|
- Which of the least bond angle ?
Text Solution
|
- In the compound 'X', all the bond angles are exactly 109^(@)28. 'X' ma...
Text Solution
|
- Cl-P-Cl bond angles in PCl5 molecule are
Text Solution
|
- The bond angle and % of d-character in SF(6) are
Text Solution
|
- Which of the following species has tetrahedral geometry?
Text Solution
|
- The bond angle in water molecule is nearly Or Directed bonds in water ...
Text Solution
|
- In which pair of species both species do have the similar geometry ? .
Text Solution
|
- The C-H bond distance is the longest in:
Text Solution
|
- Which of the following molecule is planar?
Text Solution
|
- As compared to pure atomic orbitals, hybrid orbitals have
Text Solution
|
- In ethene, the bond angles are exactly
Text Solution
|
- The percentage of p character of hybrid orbitals in graphite and diamo...
Text Solution
|
- In which of the following molecules /ions , are all the bonds not equa...
Text Solution
|
- Compound having planar symmetry is
Text Solution
|
- The structure and hybridisation of Si in Si(CH(3))(4) is
Text Solution
|
- If molecule MX3 has zero dipole moment, the sigma bonding orbitals use...
Text Solution
|