A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Dipole Moment)|25 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Polarisation and Fajan.s Rule)|14 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (VSEPR Theory)|46 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-NCERT BASED QUESTIONS (Hybridisation)
- Benzene ring has alternate single and double bonds, yet all the C-C bo...
Text Solution
|
- The pair of species having identical shapes for molecules of both spec...
Text Solution
|
- Among the following compounds the one that is polar and has central at...
Text Solution
|
- The valency of carbon in four. On what principle it can be explained i...
Text Solution
|
- In a regular octahedral molecule MX(6) the number of X - M - X bonds ...
Text Solution
|
- HgCl(2) is a solid containing
Text Solution
|
- Likely bond angles of SF(4) molecule are :
Text Solution
|
- Maximum bond angle is present in case of
Text Solution
|
- Two types of FXF angles are present in which of the following molecule...
Text Solution
|
- In which of the following molecules are all the bonds not equal ?
Text Solution
|
- In diborane, the H-B-H bond angle is 120^(@). The hybridization of bor...
Text Solution
|
- The d-orbital involved in sp^3d hybridisation is
Text Solution
|
- In graphite, electrons are
Text Solution
|
- The bond angle in PH3 is :
Text Solution
|
- Which of the following is not linear
Text Solution
|
- Deaw the structure of BrF3 molecule.
Text Solution
|
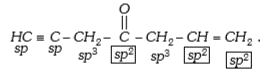
- The number of sp^2 hybridized carbon atoms in HC -= C - CH2 - overs...
Text Solution
|
- The numbers of lone pair and bond pairs in hydrazine are, respectively...
Text Solution
|
- The order of electronegativity of carbon in sp, sp^(2) and sp^(3) hybr...
Text Solution
|
- The shape of SCl4 is best described as a
Text Solution
|