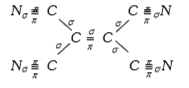
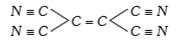A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- How many simga and pi bonds are there in the molecule of tetracyanoet...
Text Solution
|
- How many sigma bonds and pi bonds are present in a benzene molecules ?...
Text Solution
|
- How many sigma and pi bonds are in the molecule of tetracyanoethylene
Text Solution
|
- How many sigma- and pi- bonds are there in the molecule of tetracyanoe...
Text Solution
|
- The number of sigma and pi bond s I n the molecule of tetracyanoethyle...
Text Solution
|
- Number of sigma and pi bonds present in tetracyanoethylene are
Text Solution
|
- How many sigma and pi-bonds are there in a molecule of acetylene ?
Text Solution
|
- How many (pi) -bonds are there in the nitrogen molecule ?
Text Solution
|
- How many sigma and pi bonds are there in the following molecule. et...
Text Solution
|