A
B
C
D
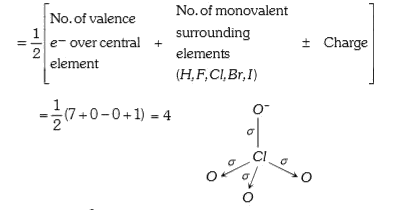
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- All the four sigma bonds in perchlorate ion are
Text Solution
|
- The nature of pi-bonds in perchlorate ion
Text Solution
|
- All the four sigma bonds in perchlorate ion are
Text Solution
|
- Cl-O bond order in perchlorate ion is
Text Solution
|
- परक्लोरेट आयन में सभी चार सिग्मा बन्ध होते हैं :
Text Solution
|
- The total number of sigma- bonds in P(6)O(18)^(6-) ion is :
Text Solution
|
- " In perchlorate ion,the "pi" - bonds are of the type."
Text Solution
|
- In perchlorate ion,the pi -bonds are of the type
Text Solution
|
- The number of Cl=O bonds is perchloric acid is , "".
Text Solution
|