A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
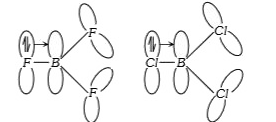
- The Lewis acidity of BF3 is less than BCl3 even though fluorine is mor...
Text Solution
|
- Fluorine is more electronegative than chlorine but p-fluorobenzoic aci...
Text Solution
|
- Assertion: The electron affinity of chlorine is greater than that of f...
Text Solution
|
- Fluorine is more electronegative than chlorine. Is the statement true ...
Text Solution
|
- Statement-1 : Fluorine is more electronegative than chlorine. Statemen...
Text Solution
|
- Fluorine is more electronegative than chlorine but p-fluorobenzoic aci...
Text Solution
|
- BF3 is a weaker Lewis acid than BCl3 , even though F is more electrone...
Text Solution
|
- BCl3 is a Lewis acid than BF3 .
Text Solution
|
- BF3 is a weaker Lewis acid than BCl3 -why?
Text Solution
|