A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Polarisation and Fajan.s Rule)|14 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Resonance)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Hybridisation)|67 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-NCERT BASED QUESTIONS (Dipole Moment)
- In a polar molecule , the ionic charge is 4.8 xx 10^(-10) esu. If the ...
Text Solution
|
- If the molecule of HCl were totally polar, the observed value of dipol...
Text Solution
|
- Given : Dipole moment of HCl=1.03 D Bond length =127pm Dipole mom...
Text Solution
|
- The dipole moment of chlorobenzene is 1.73 D . The dipole moment of p-...
Text Solution
|
- Dipole-dipole forces act between the molecules possessing permanent di...
Text Solution
|
- Which of the following will show least dipole character ?
Text Solution
|
- Which of the following would have a permanent dipole moment ?
Text Solution
|
- Among the following the molecule possessing highest dipole moment is
Text Solution
|
- Zero dipole moment is present in
Text Solution
|
- The electronegaivity difference between N and F is greater than that b...
Text Solution
|
- Fluorine is more electronegative than either boron or phosphorous. Wha...
Text Solution
|
- N(2) is less reactive than CN^(-) due to
Text Solution
|
- Which of the following fluorides of Xe has zero dipole moment ?
Text Solution
|
- Dipole moment of is 1.5D. The dipole moment of
Text Solution
|
- Dipole-dipole interaction energy between polar molecules in solids dep...
Text Solution
|
- Arrange the following compounds in order of increasing dipole moment: ...
Text Solution
|
- Polarity in a molecule and hence the dipole moment depends primarily o...
Text Solution
|
- CO is practically non-polar since
Text Solution
|
- The moecule with non-zero dipole moment is -
Text Solution
|
- The molecule with the highest dipole moment among the following is
Text Solution
|
 is 1.5D. The dipole moment of
is 1.5D. The dipole moment of 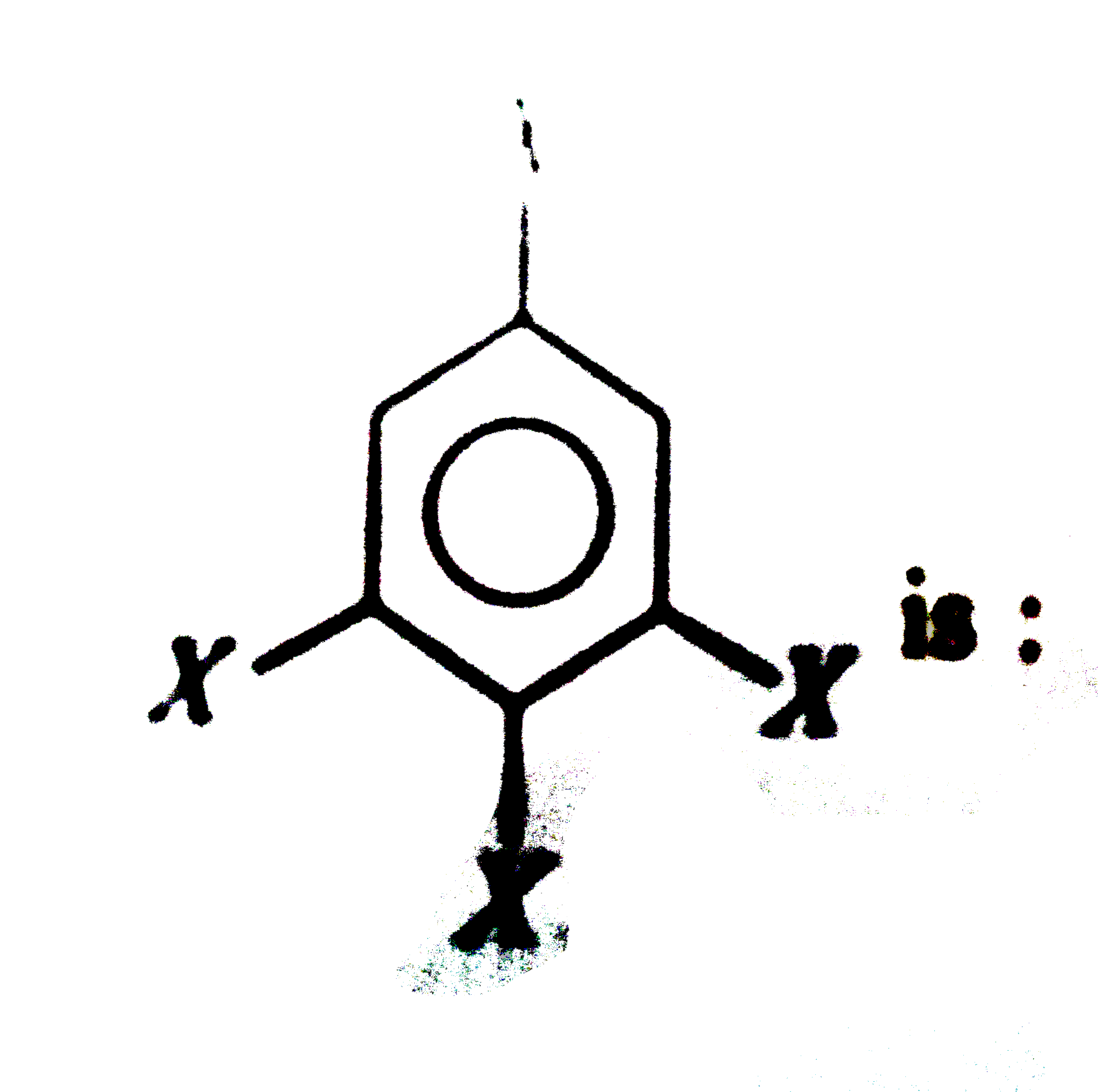
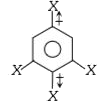 , The bond moments at para positions cancel due to opposite direction. The other two groups are meta to each other, thus angle between the two bond moments is 120° and hence,`mu = 1.5D.`
, The bond moments at para positions cancel due to opposite direction. The other two groups are meta to each other, thus angle between the two bond moments is 120° and hence,`mu = 1.5D.`