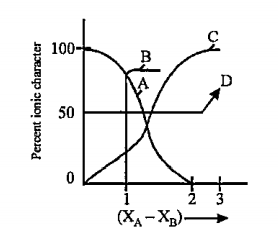
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Resonance)|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Molecular Orbital Theory)|43 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Dipole Moment)|25 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-NCERT BASED QUESTIONS (Polarisation and Fajan.s Rule)
- The polarizing power of the following anions \(N^{3-}\),\(O^{2-}\) and...
Text Solution
|
- The bonds between P atoms and Cl atoms in PCl5 are likely to be
Text Solution
|
- Maximum covalent character is associated with the compound
Text Solution
|
- Choose the correct statement
Text Solution
|
- Pauling's electronegativity values for elements are useful in predicti...
Text Solution
|
- Which among the following solids is a non-polar solid ?
Text Solution
|
- The correct order of the increasing ionic character is
Text Solution
|
- Correct order of polarising power is
Text Solution
|
- The potarising ability of which one of the following is the highest
Text Solution
|
- Which of the following statements is correct
Text Solution
|
- Amongst LiCl, RbCl, BeCl2 and MgCl2, the compounds whith the greatrest...
Text Solution
|
- For AB bond if percent ionic character is plotted against electro neha...
Text Solution
|
- For BCl(3), AlCl(3) and GaCl(3) the increasing order of ionic characte...
Text Solution
|
- The values of electronegativity of atom A and B are 1.20 and 4.0 respe...
Text Solution
|