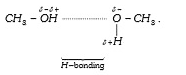
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Types of Bonding and Forces in Solid)|13 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise PAST YEARS QUESTIONS|74 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
ERRORLESS|Exercise NCERT BASED QUESTIONS (Molecular Orbital Theory)|43 VideosCHEMICAL EQUILIBRIUM
ERRORLESS|Exercise ASSERTION & REASON |12 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-NCERT BASED QUESTIONS (Hydrogen Bonding)
- What is the dominant intermolecular forces or bond that must be overco...
Text Solution
|
- The reason for exceptionally high boiling point of water is
Text Solution
|
- Which of the following has the strongest H - bond?
Text Solution
|
- H2 O is a liquid while H2 S is a gas.
Text Solution
|
- Which one among the following does not have the hydrogen bond?
Text Solution
|
- which of the following hydrogen bond is strongest in vapour phase ?
Text Solution
|
- In which of the following substances will hydrogen bond be strongest ?
Text Solution
|
- The hydrogen bonds are encountered in HF, H2 O, NH3 and HF2^-. The rel...
Text Solution
|
- When two ice cubes are pressed over each other, they unite to form one...
Text Solution
|
- H-bonding is maximum in
Text Solution
|
- Which of the following is sparingly soluble in water ?
Text Solution
|
- o-nitrophenol is more volatile than p-nitrophenol it is due to
Text Solution
|
- At room temperature, HCl is a gas while HF is low boiling liquid. This...
Text Solution
|
- The maximum possible number of hydrogen bonds in which an H(2)O molecu...
Text Solution
|
- Hydrogen bonding is maximum in
Text Solution
|
- Which one has the highest boiling point
Text Solution
|
- The high density of water compared to ice is due to
Text Solution
|
- Hydrogen bonds are formed in many compounds e.g. H(2)O, HF, NH(3). The...
Text Solution
|
- How many hydrogen-bonded water molecule(s) are associated in CuSO(4).5...
Text Solution
|
- When we move from HF to HCl , the boiling point sharply but on moving...
Text Solution
|