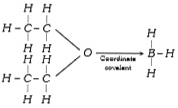
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-PAST YEARS QUESTIONS
- Which of the following contains a coordinate and covalent bond
Text Solution
|
- What type of bonds are formed in N2O4 and what is the name of this com...
Text Solution
|
- What is the nature of the bond between B and O in (C(2)H(5))(2)OBH(3)
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Consider the molecules CH(4),NH(3) and H(2)O which of the given statem...
Text Solution
|
- Predict the correct order of repulsions among the following :
Text Solution
|
- Which of the following is not a correct statement?
Text Solution
|
- In which of the following pairs, the two spices are iso-structural?
Text Solution
|
- In BrF(3) molecule, the lone pairs occupy equatorial positions to mini...
Text Solution
|
- Sulphur reacts with chlorine in 1:2 ration and forms X . Hydrolysis of...
Text Solution
|
- Match the interhalogen compounds of column I with the geometry in colu...
Text Solution
|
- In the structure of ClF(3), the number of lone pairs of electrons on c...
Text Solution
|
- Identify the incorrect statement related to PCl(5) from the follwing
Text Solution
|
- The correct structure of tribromootaoxide.
Text Solution
|
- In which of the following molecules/ions in the central atom sp^2-hybr...
Text Solution
|
- In HCHO , 'C' has hybridization
Text Solution
|
- Which of the two lons from the list given have the geometry that is ex...
Text Solution
|
- Wen a hybridization state of carbon atom changes from sp^(3) to sp^(2)...
Text Solution
|
- In which one of the following species the central atom has the type of...
Text Solution
|
- In which of the following molecules, the central atom does not have sp...
Text Solution
|