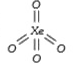
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-PAST YEARS QUESTIONS
- In which of the following molecules, the central atom does not have sp...
Text Solution
|
- Some of the properties of the two species, NO(3)^(-) and H(3)O^(+) are...
Text Solution
|
- Identify the incorrect statements, regarding the molecule XeO(4)
Text Solution
|
- In which of the following pair both the species have sp^(3) hybridizat...
Text Solution
|
- XeF2 is isostructural with
Text Solution
|
- Which one of the following species has plane triangular shape ?
Text Solution
|
- The correct geometry and hybridization for XeF4 are
Text Solution
|
- Which of the following statement is not correct
Text Solution
|
- Which among the following possesses an sp hybridized carbon in its str...
Text Solution
|
- In which of the following pairs, both the species are not isostructura...
Text Solution
|
- The structural formula of a compound is CH(3)-CH=C=CH(2). The type of ...
Text Solution
|
- Out of SO2, BeCl2, O3, H2O and HgCl2, the linear species are
Text Solution
|
- The smallest bond angle around the central atom will be there in
Text Solution
|
- Match the compounds given in Column I with the hybridisation and shape...
Text Solution
|
- In an octahedral structure , the pair of d orbitals involved in d^(2)...
Text Solution
|
- Planar structure is shown by
Text Solution
|
- Which of the following molecules represents the order of hybridisation...
Text Solution
|
- The outer orbitals of C in ethene molecule can be considered to be hyb...
Text Solution
|
- Which one of the following molecules contains no pi bond?
Text Solution
|
- Which of the following species contains equal number of pi and pi bond...
Text Solution
|