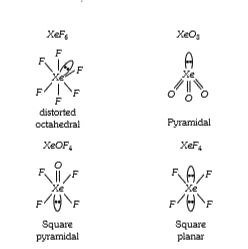
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-PAST YEARS QUESTIONS
- Out of SO2, BeCl2, O3, H2O and HgCl2, the linear species are
Text Solution
|
- The smallest bond angle around the central atom will be there in
Text Solution
|
- Match the compounds given in Column I with the hybridisation and shape...
Text Solution
|
- In an octahedral structure , the pair of d orbitals involved in d^(2)...
Text Solution
|
- Planar structure is shown by
Text Solution
|
- Which of the following molecules represents the order of hybridisation...
Text Solution
|
- The outer orbitals of C in ethene molecule can be considered to be hyb...
Text Solution
|
- Which one of the following molecules contains no pi bond?
Text Solution
|
- Which of the following species contains equal number of pi and pi bond...
Text Solution
|
- Among the following which one is wrong statement ?
Text Solution
|
- Which molecules has zero dipole moment ?
Text Solution
|
- Which bond angle, theta would result in the maximum dipole moment for...
Text Solution
|
- H2O is dipolar, whereas BeF2 is not. It is because
Text Solution
|
- Which of the following has the highest dipole moment?
Text Solution
|
- Which of the following arrangment of molecules is correct on the basi...
Text Solution
|
- Dipole-induced dipole interaction are present in which of the followin...
Text Solution
|
- Which of the following molecules has the maximum dipole moment ?
Text Solution
|
- Which of the following is the most polar?
Text Solution
|
- If the electron pair forming a bond between two atoms and B is not in ...
Text Solution
|
- Which of the following have both polar and non-polar bonds?
Text Solution
|