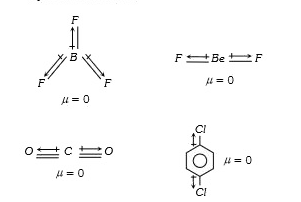
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-PAST YEARS QUESTIONS
- Which of the following is a polar compound ?
Text Solution
|
- Which of the following is a polar molecule
Text Solution
|
- Which of the following species does not exist under normal conditions?
Text Solution
|
- According to MO theory which of thhe following lists makes the nitroge...
Text Solution
|
- Four diatomic species are listed below in different sequences. Which o...
Text Solution
|
- Which of the following is not paramagnetic?
Text Solution
|
- Which of the following molecule has highest bond energy?
Text Solution
|
- The species having bond angle of 120^(@) is
Text Solution
|
- Which one of the following pairs of species have the same bond order ?
Text Solution
|
- Consider the following species CN^(-),CN^(-),NO and CN. Which one ...
Text Solution
|
- Which of the following diatomic molecular species has only pi bonds ac...
Text Solution
|
- In which of the following compounds does hydrogen bonding occur
Text Solution
|
- The boiling point of ethanol is higher as compared to the boiling of d...
Text Solution
|
- Which of the following compound has the highest boiling point
Text Solution
|
- Which one of the following compounds shows the presence of intramolecu...
Text Solution
|
- Identify a molecule which does not exist.
Text Solution
|
- Which of the following set of molecules will have zero diplole moment?
Text Solution
|
- BF3 is planar and electron deficient compound. Hybridization and numbe...
Text Solution
|
- Match List - I with List - II. Choose the correct answer from th...
Text Solution
|
- Which of the following molecules is non-polar in nature?
Text Solution
|