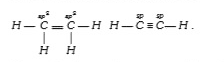A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Assertion & Reason
- Assertion : Water is a good solvent for ionic compounds but poor one...
Text Solution
|
- Assertion : The atoms in a covalent molecule are said to share el...
Text Solution
|
- Assertion : Diborane is electron deficient. Reason: There are no en...
Text Solution
|
- A resonance hybrid is always more stable than any of its canonical str...
Text Solution
|
- Assertion : All F - S - F angle in SF(4) are greater than 90^(@) but ...
Text Solution
|
- Assertion. C-H bond in ethyne is shorter than C-H bonds in ethene. ...
Text Solution
|
- Assertion: Nitrogen molecule is diamagnetic. Reason: N2 molecule hav...
Text Solution
|
- Assertion : Ice is less dense than liquid water. Reason: There are v...
Text Solution
|
- Assertion: Iodine is more soluble in C CI(4) than in water. Reason: ...
Text Solution
|
- Assertion: o-and p-nitrophenol can be separated by steam distillation....
Text Solution
|
- Assertion: Bond energy has order like C-C lt C=C lt C -= C. Reason: ...
Text Solution
|
- Assertion : B(2) molecule is paramagnetic. Reason :The highest occup...
Text Solution
|
- Assertion : The nearly tetrahedral arrangement of the orbitals about t...
Text Solution
|
- Assertion :The S-S-S bond in S(8) molecule is 105^(@). Reason :S(8)...
Text Solution
|
- Assertion(A) - Fluorine molecule has bond order one. Reason(R)-The n...
Text Solution
|
- Assertion : H-S-H bond angle in H(2)S is closer to 90^(@) but H-O-H b...
Text Solution
|
- Assertion : All F - S - F angle in SF(4) are greater than 90^(@) but ...
Text Solution
|