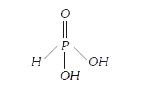A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
ERRORLESS|Exercise NCERT BASED QUESTIONS (COMMON ION EFFECT, ISOHYDRIC SOLUTIONS, SOLUBILITY PRODUCT, IONIC PRODUCT OF WATER AND SALT HYDROLYSIS)|59 VideosIONIC EQUILIBRIUM
ERRORLESS|Exercise NCERT BASED QUESTIONS (HYDROGEN ION CONCENTRATION- PH SCALE AND BUFFER SOLUTION)|110 VideosIONIC EQUILIBRIUM
ERRORLESS|Exercise ASSERTION AND REASON |9 VideosHYDROGEN
ERRORLESS|Exercise ASSERTION & REASON|6 VideosPURIFICATION, CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
ERRORLESS|Exercise ASSERTION & REASON|9 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-IONIC EQUILIBRIUM-NCERT BASED QUESTIONS (ACIDS AND BASES)
- Which one of the following is called amphoteric solvent
Text Solution
|
- Which of the following is the strongest conjugate base ?
Text Solution
|
- The basicity of phosphorus acid (H(3)PO(3)) is ............ .
Text Solution
|
- The conjugate acid of NH(2)^(-) is :
Text Solution
|
- The strength of an acid depends on its tendency to
Text Solution
|
- The strongest base of the following species is
Text Solution
|
- The solvent which neither accepts proton nor donates proton is called
Text Solution
|
- Strongest conjugate base is
Text Solution
|
- Which one is the weakest acid
Text Solution
|
- The correct order of acid strength is
Text Solution
|
- The strongest Bronsted base in the following anion is
Text Solution
|
- pK(a) values of two acids. A and B are 4 and 5. The strengths of these...
Text Solution
|
- The relative basic character of the following is
Text Solution
|
- Which one is the strongest acid
Text Solution
|
- Arrange NH(4)^(+), H(2)O, H(3)O^(+), HF and OH^(-) in increasing order...
Text Solution
|
- According to Bronsted Lowry concept, the correct order of strength of ...
Text Solution
|
- With reference to protonic acids, which of the following statements is...
Text Solution
|
- The correct order of acidity for the following is
Text Solution
|
- The strongest acid is
Text Solution
|
- Increasing order of acidic character would be
Text Solution
|